Aromatic-turmerone's anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling.
Sun Young Park, Young Hun Kim, YoungHee Kim, Sang-Joon Lee
文献索引:Neurochem. Int. 61(5) , 767-77, (2012)
全文:HTML全文
摘要
Despite data supporting an immune-modulating effect of ar-turmerone in vitro, the underlying signaling pathways are largely unknown. Here, we investigated the anti-neuroinflammatory properties of ar-turmerone in LPS-stimulated BV-2 microglial cells. Increased pro-inflammatory cytokines and chemokines, PGE(2), NO and ROS production and MMP-9 enzymatic activity in LPS-stimulated microglial cells was inhibited by ar-turmerone. Subsequent mechanistic studies revealed that ar-turmerone inhibited LPS-induced JNK, p38 MAPK and NF-κB activation. Furthermore, ar-turmerone decreased the phosphorylation of LPS-induced STAT-1. Additionally, ar-turmerone increased the phosphorylation of STAT-3, an anti-inflammatory transcription factor. We next demonstrated that ar-turmerone induced HO-1 and Nrf-2 activation suppressed the activation of neuroinflammatory molecules in LPS-induced microglial cells, and that down-regulation of HO-1 signals was sufficient to induce the expression of iNOS, COX-2 and ROS production in microglial cells. Interestingly, we found that ar-turmerone induced phosphorylation of CREB by upregulating the cAMP level in microglial cells. Furthermore, HO-1 activation via PKA-mediated CREB phosphorylation attenuated the expression of neuroinflammatory molecules in LPS-induced microglial cells. Overall, the results of this study demonstrate that HO-1 and its upstream effectors PKA play a pivotal role in the anti-neuroinflammatory response of ar-turmerone in LPS-stimulated microglia.Copyright © 2012 Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
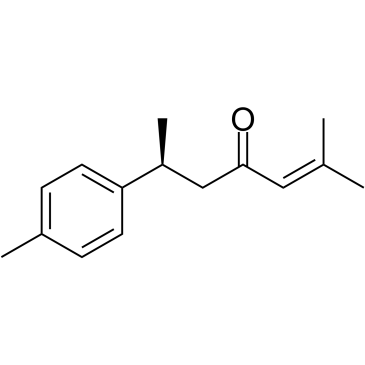 |
芳姜黄酮
CAS:532-65-0 |
C15H20O |
|
Turmeric (Curcuma longa L.) volatile oil inhibits key enzyme...
2012-11-01 [Int. J. Food Sci. Nutr. 63(7) , 832-4, (2012)] |
|
Selective induction of apoptosis by ar-turmerone isolated fr...
2002-05-01 [Int. J. Mol. Med. 9(5) , 481-4, (2002)] |
|
Induction of apoptosis by ar-turmerone on various cell lines...
2004-08-01 [Int. J. Mol. Med. 14(2) , 253-6, (2004)] |
|
Sesquiterpenoids from the rhizome of Curcuma zedoaria.
2001-10-01 [Arch. Pharm. Res. 24(5) , 424-6, (2001)] |
|
Activation of apoptotic protein in U937 cells by a component...
2009-02-28 [BMB Rep. 42(2) , 96-100, (2009)] |