Histone 2A stimulates glucose-6-phosphatase activity by permeabilization of liver microsomes.
Angelo Benedetti, Rosella Fulceri, Bernard B Allan, Pamela Houston, Andrey L Sukhodub, Paola Marcolongo, Brian Ethell, Brian Burchell, Ann Burchell
文献索引:Biochem. J. 367(Pt 2) , 505-10, (2002)
全文:HTML全文
摘要
Histone 2A increases glucose-6-phosphatase activity in liver microsomes. The effect has been attributed either to the conformational change of the enzyme, or to the permeabilization of microsomal membrane that allows the free access of substrate to the intraluminal glucose-6-phosphatase catalytic site. The aim of the present study was the critical reinvestigation of the mechanism of action of histone 2A. It has been found that the dose-effect curve of histone 2A is different from that of detergents and resembles that of the pore-forming alamethicin. Inhibitory effects of EGTA on glucose-6-phosphatase activity previously reported in histone 2A-treated microsomes have been also found in alamethicin-permeabilized vesicles. The effect of EGTA cannot therefore simply be an antagonization of the effect of histone 2A. Histone 2A stimulates the activity of another latent microsomal enzyme, UDP-glucuronosyltransferase, which has an intraluminal catalytic site. Finally, histone 2A renders microsomal vesicles permeable to non-permeant compounds. Taken together, the results demonstrate that histone 2A stimulates glucose-6-phosphatase activity by permeabilizing the microsomal membrane.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
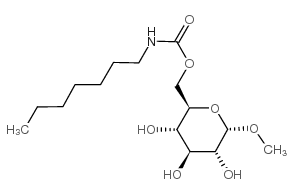 |
6-O-(N-庚甲酰)-甲基-α-D-葡萄糖苷
CAS:115457-83-5 |
C15H29NO7 |
|
Atorvastatin increases lipopolysaccharide-induced expression...
2014-06-01 [Exp. Ther. Med. 8(1) , 219-228, (2014)] |
|
Synthesis and characterization of 6-O-(N-heptylcarbamoyl)-me...
1989-05-15 [Anal. Biochem. 179 , 145, (1989)] |
|
Pif1 family helicases suppress genome instability at G-quadr...
2013-05-23 [Nature 497 , 458-62, (2013)] |
|
Stabilization of integral membrane proteins in aqueous solut...
1998-01-01 [Biochimie 80(5-6) , 515-30, (1998)] |
|
Comparative extraction procedures for a galactose-specific l...
1998-01-01 [Appl. Microbiol. Biotechnol. 49(1) , 16-23, (1998)] |