Tröger's base. An alternate synthesis and a structural analog with thromboxane A2 synthetase inhibitory activity.
R A Johnson, R R Gorman, R J Wnuk, N J Crittenden, J W Aiken
文献索引:J. Med. Chem. 36(21) , 3202-6, (1993)
全文:HTML全文
摘要
The synthesis of 2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (Tröger's base) from p-toluidine and of two Tröger's base analogs from other anilines by reaction with hexamethylenetetramine in trifluoroacetic acid is described. 2,3,6,7-Tetrahydro-9-methyl-2,6-di-p-tolyl-1H,5H-pyrimido[5,6,1-ij] quinazoline is formed as a secondary product in the reaction of p-toluidine and hexamethylenetetramine. One of the Tröger's base analogs, 2,8-bis(3'-pyridylmethyl)-6H,12H-5,11-methanodibenzo[b,f][1,5]d iazocine (5), is an effective inhibitor of the enzyme, thromboxane A2 (TxA2) synthase, with an ED50 of 30 ng/mL in a specified in vitro assay. Three analogs having substituents on the bridging methylene group of the bicyclic nucleus of the Tröger's base structure were prepared, but all were considerably less active than the aforementioned compound in the inhibition assay. The structures of these inhibitors of TxA2 synthase fall outside the classical structure-activity relationship that has been established for this class of enzyme inhibitors.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
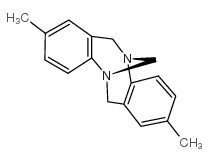 |
朝格尔碱
CAS:529-81-7 |
C17H18N2 |
|
Tröger's base-functionalised organic nanoporous polymer for ...
2010-02-14 [Chem. Commun. (Camb.) 46(6) , 970-2, (2010)] |
|
Intermittent simulated moving bed chromatography: 3. Separat...
2011-12-30 [J. Chromatogr. A. 1218(52) , 9345-52, (2011)] |
|
Tröger's-base-derived infinite co-ordination polymer micropa...
2009-01-01 [Small 5(1) , 46-50, (2009)] |
|
Enantioseparation of tetrahydropalmatine and Tröger's base b...
2007-02-23 [J. Biochem. Biophys. Methods 70(1) , 71-6, (2007)] |
|
Optically active Ru(II) complexes with a chiral Tröger's bas...
2007-07-01 [J. Inorg. Biochem. 101(7) , 987-96, (2007)] |