Structure-activity relationship and elucidation of the determinant factor(s) responsible for the mechanism-based inactivation of cytochrome P450 2B6 by substituted phenyl diaziridines.
Yoshimasa Kobayashi, Chitra Sridar, Ute M Kent, Satish G Puppali, John M Rimoldi, Haoming Zhang, Lucy Waskell, Paul F Hollenberg
文献索引:Drug Metab. Dispos. 34(12) , 2102-10, (2006)
全文:HTML全文
摘要
It has been demonstrated previously that several 3-trifluoromethyl-3-(4-alkoxyphenyl)diaziridines inhibit the 7-ethoxy-4-(trifluoroethyl)coumarin (7-EFC) O-deethylation activity of P450 2B6 in a mechanism-based manner. In contrast, 3-trifluoromethyl-3-(4-methylthio)phenyl)diaziridine did not have any effect on the activity of P450 2B6. It is interesting that both the alkoxy and the thiophenyl compounds were metabolized by P450 2B6. In this report, the structure-activity relationships for the mechanism-based inactivation of cytochrome P450 2B6 by a series of aryl diaziridines were investigated. Three diaziridines that did not contain a 4-alkoxy-substituent on their phenyl ring, namely, 3-trifluoromethyl-3-(3-methoxyphenyl)diaziridine, 3-trifluoromethyl-3-phenyl diaziridine, and 3-trifluoromethyl-3-(4-chlorophenyl)diaziridine had no effect on the P450 2B6 7-EFC activity. Another analog that did not contain a diaziridine substructure, 3-trifluoromethyl-3-(4-methoxyphenyl)ethanone, also had no effect on the activity of P450 2B6. Glutathione ethyl ester adducts of the phenyldiaziridine reactive intermediates were isolated from reaction mixtures of the inactivated samples and analyzed by liquid chromatography-tandem mass spectrometry. The structures of the conjugates suggested that the electrophilic reactive intermediate in each case was a quinone methide (quinomethane), 4-ethylidene-cyclohexa-2,5-dienone, generated from the 4-alkoxyphenyldiaziridines by removal of both of the diaziridine and the 4-alkyl groups. In conclusion, the determinant factor for the mechanism-based inactivator activity of the aryl diaziridines seems to be the formation of the reactive quinomethane intermediate, which is generated from the 4-alkoxyphenyl diaziridines by a cytochrome P450-catalyzed metabolic reaction.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
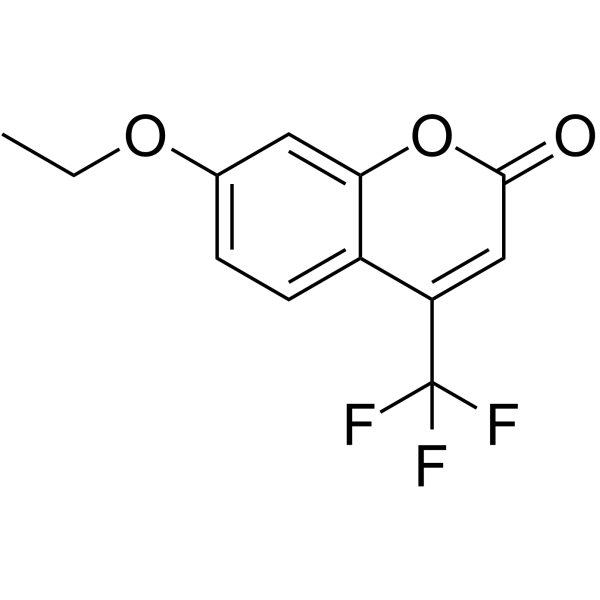 |
7-乙氧基-4-(三氟甲基)香豆素
CAS:115453-82-2 |
C12H9F3O3 |
|
HepG2 cells as an in vitro model for evaluation of cytochrom...
2015-01-01 [Arch. Pharm. Res. 38 , 691-704, (2015)] |
|
Environmentally persistent free radical-containing particula...
2015-12-01 [Toxicol. Appl. Pharmacol. 289 , 223-30, (2015)] |
|
Inhibition of cytochrome P450 2B4 by environmentally persist...
2015-05-15 [Biochem. Pharmacol. 95 , 126-32, (2015)] |
|
Effects of artemisinin antimalarials on Cytochrome P450 enzy...
2014-07-01 [Xenobiotica 44(7) , 615-26, (2014)] |
|
Interaction of selected platinum(II) complexes containing ro...
2015-09-01 [Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 159 , 382-7, (2015)] |