Phase 1 safety and immunogenicity trial of the Plasmodium falciparum blood-stage malaria vaccine AMA1-C1/ISA 720 in Australian adults.
Mark A Pierce, Ruth D Ellis, Laura B Martin, Elissa Malkin, Eveline Tierney, Kazutoyo Miura, Michael P Fay, Joanne Marjason, Suzanne L Elliott, Gregory E D Mullen, Kelly Rausch, Daming Zhu, Carole A Long, Louis H Miller
文献索引:Vaccine 28(10) , 2236-42, (2010)
全文:HTML全文
摘要
A Phase 1 trial was conducted in malaria-naïve adults to evaluate the recombinant protein vaccine apical membrane antigen 1-Combination 1 (AMA1-C1) formulated in Montanide ISA 720 (SEPPIC, France), a water-in-oil adjuvant. Vaccinations were halted early due to a formulation issue unrelated to stability or potency. Twenty-four subjects (12 in each group) were enrolled and received 5 or 20 microg protein at 0 and 3 months and four subjects were enrolled and received one vaccination of 80 microg protein. After first vaccination, nearly all subjects experienced mild to moderate local reactions and six experienced delayed local reactions occurring at Day 9 or later. After the second vaccination, three subjects experienced transient grade 3 (severe) local reactions; the remainder experienced grade 1 or 2 local reactions. All related systemic reactogenicity was grade 1 or 2, except one instance of grade 3 malaise. Anti-AMA1-C1 antibody responses were dose dependent and seen following each vaccination, with mean antibody levels 2-3 fold higher in the 20 microg group compared to the 5 microg group at most time points. In vitro growth-inhibitory activity was a function of the anti-AMA1 antibody titer. AMA1-C1 formulated in ISA 720 is immunogenic in malaria-naïve Australian adults. It is reasonably tolerated, though some transient, severe, and late local reactions are seen.Published by Elsevier Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
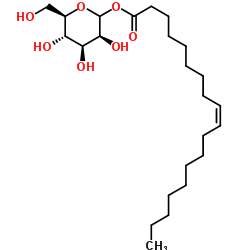 |
甘露醇油酸酯
CAS:9049-98-3 |
C24H44O7 |
|
Protection against an intranasal challenge by vaccines formu...
2009-08-06 [Vaccine 27(36) , 5020-5, (2009)] |
|
Immunization with pre-erythrocytic antigen CelTOS from Plasm...
2010-01-01 [PLoS ONE 5(8) , e12294, (2010)] |
|
Effects on immunogenicity by formulations of emulsion-based ...
2012-10-01 [Clin. Vaccine Immunol. 19(10) , 1633-40, (2012)] |
|
Pichia pastoris-expressed dengue virus type 2 envelope domai...
2010-07-01 [J. Virol. Methods 167(1) , 10-6, (2010)] |
|
CpG oligodeoxynucleotide and montanide ISA 206 adjuvant comb...
2011-10-19 [Vaccine 29(45) , 7960-5, (2011)] |