| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
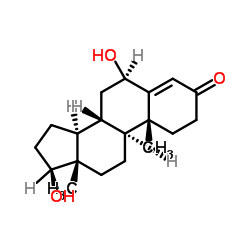 |
6β-羟基睾酮与甲醇的配制溶液
CAS:62-99-7 |
|
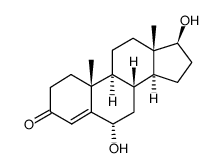 |
6alpha-羟基睾酮
CAS:2944-87-8 |