anticonvulsant and toxicity evaluation of newer 4H-benzo[1,4]oxazin-3-ones: The effect of two hydrogen bonding domains.
Nadeem Siddiqui, Ruhi Ali, M Faiz Arshad, Waquar Ahsan, Sharique Ahmed, M Shamsher Alam
文献索引:Arch. Pharm. (Weinheim) 343(11-12) , 657-63, (2010)
全文:HTML全文
摘要
A series of (Z)-2-(substituted aryl)-N-(3-oxo-4-(substituted carbamothioyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-yl) hydrazine carboxamides (6a-r) was synthesized using 2-amino-5-nitrophenol as a starting material. All the synthesized compounds possessed two hydrogen-bonding domains and their effect on the activity was studied thereof. The anticonvulsant activity was assessed by the maximal electroshock test (MES), subcutaneous pentylenetetrazole test (scPTZ) and intraperitoneal thiosemicarbazide test (ipTSC). Compounds (6b, 6h, 6i, and 6p) were found to be the most potent of the series as they showed 83-100% protection in the MES test. They also displayed considerable activity in the chemically induced seizure tests. Most of the tested compounds were devoid of the neurotoxic and hepatotoxic effects.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
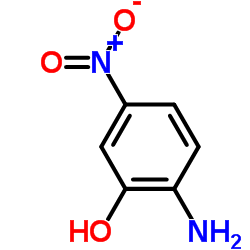 |
2-氨基-5-硝基苯酚
CAS:121-88-0 |
C6H6N2O3 |
|
Effect of gradient steepness on the kinetic performance limi...
2015-08-28 [J. Chromatogr. A. 1409 , 152-8, (2015)] |
|
Final report on the safety assessment of amino nitrophenols ...
2009-01-01 [Int. J. Toxicol. 28(6 Suppl 2) , 217S-51S, (2009)] |
|
[Simultaneous determination of 11 aminophenols in hair dyes ...
2012-09-01 [Se Pu 30(9) , 870-5, (2012)] |
|
Synthesis of novel 7-benzylamino-2H-1,4-benzoxazin-3(4H)-one...
2008-06-01 [Eur. J. Med. Chem. 43 , 1216-21, (2008)] |
|
2-Amino-5-nitrophenol.
1993-01-01 [IARC Monogr. Eval. Carcinog. Risks Hum. 57 , 177-84, (1993)] |