Inhibitory effects on mushroom tyrosinase by some alkylbenzaldehydes.
Qing-Xi Chen, Kang-Kang Song, Qin Wang, Huang Huang
文献索引:J. Enzyme Inhib. Med. Chem. 18(6) , 491-6, (2003)
全文:HTML全文
摘要
The inhibition kinetics on the diphenolase activity of mushroom tyrosinase by some alkylbenzaldehydes has been investigated. The results show that the alkylbenzaldehydes assayed can lead to reversible inhibition to the enzyme; o-tolualdehyde and m-tolualdehyde are mixed-type inhibitors and p-alkylbenzaldehydes are uncompetitive inhibitors. For the p-alkylbenzaldehydes, the inhibition potency follows the order: p-tolualdehyde < p-ethylbenzaldehyde < p-propylbenzaldehyde = p-Isopropylbenzaldehyde < p-tert-butylbenzaldehyde = p-butylbenzaldehyde < p-pentylbenzaldehyde < p-hexylbenzaldehyde > p-heptylbenzaldehyde > p-octylbenzaldehyde, indicating the hydrophobic p-alkyl group played an important role in inhibition to the enzyme. The inhibitory effects of alkylbenzaldehydes on the monophenolase activity have also been studied. The results show that o-tolualdehyde and m-tolualdehyde can lengthen the lag time and decrease the steady-state activity of the enzyme, but p-alkylbenzaldehydes only decrease the steady-state activity and do not lengthen the lag time, indicating that their inhibitory mechanisms are different.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
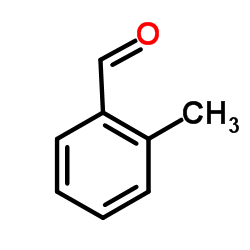 |
邻甲基苯甲醛
CAS:529-20-4 |
C8H8O |
|
Oxidation of tolualdehydes to toluic acids catalyzed by cyto...
1995-02-01 [Drug Metab. Dispos. 23(2) , 261-5, (1995)] |
|
The atmospheric photolysis of o-tolualdehyde.
2011-11-15 [Environ. Sci. Technol. 45(22) , 9649-57, (2011)] |
|
Long-term performance of passive materials for removal of oz...
2012-02-01 [Indoor Air 22(1) , 43-53, (2012)] |
|
Measurement of acid-catalyzed isomerization of unsaturated a...
[Anal. Chim. Acta 523(2) , 157-163, (2004)] |