Non-antiepileptic drugs for trigeminal neuralgia.
Mi Yang, Muke Zhou, Li He, Ning Chen, Joanna M Zakrzewska
文献索引:Cochrane Database Syst. Rev. (1) , CD004029, (2011)
全文:HTML全文
摘要
Non-antiepileptic drugs have been used in the management of trigeminal neuralgia since the 1970s.The objective was to systematically review the efficacy and tolerability of non-antiepileptic drugs for trigeminal neuralgia.For this updated review we searched the Cochrane Neuromuscular Disease Group Specialized Register (30 April 2010). We also searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2), MEDLINE (January 1966 to April 2010), EMBASE (January 1980 to April 2010), LILACS (January 1982 to April 2010) and the Chinese Biomedical Retrieval System (1978 to April 2010). We handsearched 10 Chinese journals.We searched for double-blind randomized or quasi-randomized controlled trials in which the active drug was used for at least two weeks.Two authors decided which trials fitted the inclusion criteria and independently graded risk of bias.Four trials involving 139 participants were included. The primary outcome measure in each was pain relief. Three trials with an unclear risk of bias compared one of the non-antiepileptic drugs tizanidine, tocainide or pimozide with carbamazepine. In a trial of tizanidine involving 12 participants (one dropped out due to unrelated disease) one of five treated with tizanidine and four of six treated with carbamazepine improved, risk ratio 0.30 (95% CI 0.05 to 1.89). Few side effects were noted with tizanidine. In a study involving 12 participants there was an improvement in mean pain scores with tocainide similar to that with carbamazepine, but significant side effects limited its use. In the pimozide study more participants improved on pimozide (48/48) than with carbamazepine (27/48) (risk ratio 1.76, 95% CI 1.37 to 2.26). Up to 83% of participants reported adverse effects but these did not lead to withdrawal from the study. A trial with low risk of bias involving 47 participants compared 0.5% proparacaine hydrochloride eyedrops with placebo but did not show any significant benefits or side effects.Of the four studies identified, one had low and three an unclear risk of bias. There is insufficient evidence from randomized controlled trials to show significant benefit from non-antiepileptic drugs in trigeminal neuralgia. More research is needed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
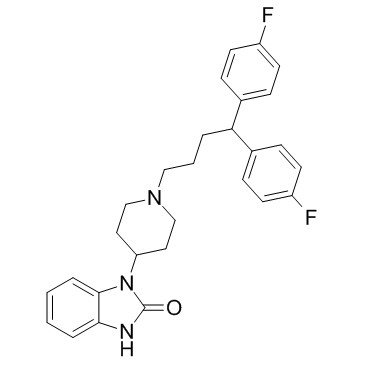 |
匹莫齐特
CAS:2062-78-4 |
C28H29F2N3O | |
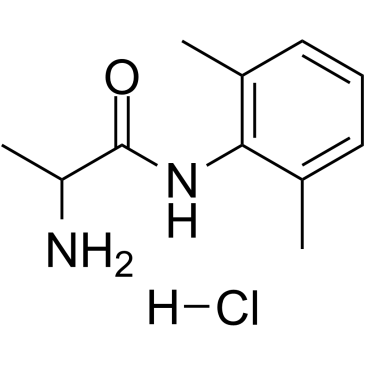 |
N-(2-氨基丙酰基)-2,6-二甲基苯胺
CAS:71395-14-7 |
C11H17ClN2O |
|
Simple and sensitive screening and quantitative determinatio...
2014-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 970 , 1-7, (2014)] |
|
Factors influencing crystal growth rates from undercooled li...
2014-08-21 [J. Phys. Chem. B 118(33) , 9974-82, (2014)] |
|
Progesterone downregulation of miR-141 contributes to expans...
2015-07-01 [Oncogene 34 , 3676-87, (2015)] |
|
Structure-Based Screen Identifies a Potent Small Molecule In...
2015-08-01 [Mol. Cancer Ther. 14 , 1777-93, (2015)] |
|
A human ether-á-go-go-related (hERG) ion channel atomistic m...
2014-11-04 [Toxicol. Lett. 230(3) , 382-92, (2014)] |