| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
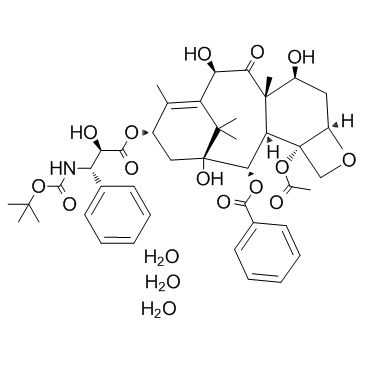 |
三水多烯紫杉醇
CAS:148408-66-6 |
|
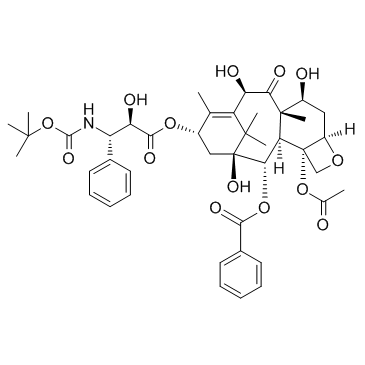 |
多烯紫杉醇;多西他赛
CAS:114977-28-5 |