An intact dilysine-like motif in the carboxyl terminus of MAL is required for normal apical transport of the influenza virus hemagglutinin cargo protein in epithelial Madin-Darby canine kidney cells.
R Puertollano, J A Martínez-Menárguez, A Batista, J Ballesta, M A Alonso
文献索引:Mol. Biol. Cell 12(6) , 1869-83, (2001)
全文:HTML全文
摘要
The MAL proteolipid, a component of the integral protein sorting machinery, has been demonstrated as being necessary for normal apical transport of the influenza virus hemagglutinin (HA) and the overall apical membrane proteins in Madin-Darby canine kidney (MDCK) cells. The MAL carboxy terminus ends with the sequence Arg-Trp-Lys-Ser-Ser (RWKSS), which resembles dilysine-based motifs involved in protein sorting. To investigate whether the RWKSS pentapeptide plays a role in modulating the distribution of MAL and/or its function in apical transport, we have expressed MAL proteins with distinct carboxy terminus in MDCK cells whose apical transport was impaired by depletion of endogenous MAL. Apical transport of HA was restored to normal levels by expression of MAL with an intact but not with modified carboxyl terminal sequences bearing mutations that impair the functioning of dilysine-based sorting signals, although all the MAL proteins analyzed incorporated efficiently into lipid rafts. Ultrastructural analysis indicated that compared with MAL bearing an intact RWKSS sequence, a mutant with lysine -3 substituted by serine showed a twofold increased presence in clathrin-coated cytoplasmic structures and a reduced expression on the plasma membrane. These results indicate that the carboxyl-terminal RWKSS sequence modulates the distribution of MAL in clathrin-coated elements and is necessary for HA transport to the apical surface.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
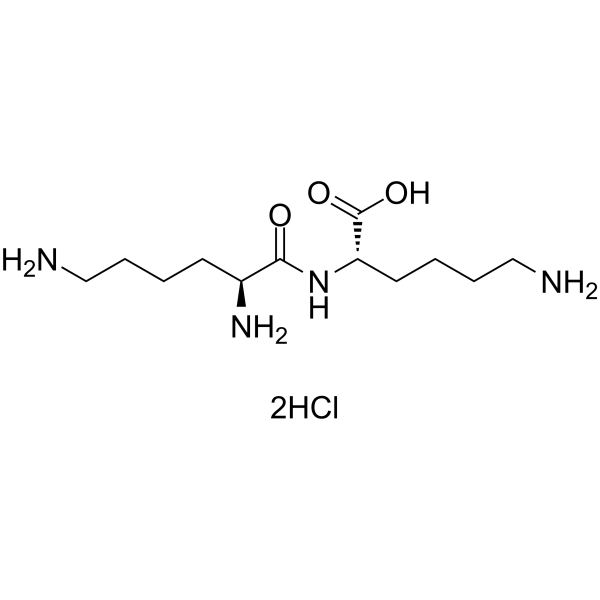 |
L-赖氨酰-L-赖氨酸二盐酸盐
CAS:52123-30-5 |
C12H28Cl2N4O3 |
|
Canine zona pellucida glycoprotein-3: up-scaled production, ...
2015-01-01 [Vaccine 33(1) , 133-40, (2014)] |
|
A single binding site for dilysine retrieval motifs and p23 ...
1998-09-29 [Proc. Natl. Acad. Sci. U. S. A. 95(20) , 11649-54, (1998)] |
|
Intracellular targeting signals contribute to localization o...
2004-06-01 [J. Virol. 78(11) , 5913-22, (2004)] |
|
Targeted gap junction protein constructs reveal connexin-spe...
2002-06-07 [J. Biol. Chem. 277(23) , 20911-8, (2002)] |
|
Peptide binding in OppA, the crystal structures of the perip...
1997-08-12 [Biochemistry 36(32) , 9747-58, (1997)] |