Reductive aldol coupling of divinyl ketones via rhodium-catalyzed hydrogenation: syn-diastereoselective construction of beta-hydroxyenones.
Soo Bong Han, Michael J Krische
文献索引:Org. Lett. 8 , 5657, (2006)
全文:HTML全文
摘要
Catalytic hydrogenation of divinyl ketones 1a and 1e in the presence of diverse aldehydes 2a-e at ambient temperature and pressure using cationic rhodium catalysts ligated by tri-2-furyl phosphine enables formation of aldol products 3a-e and 5a-e, respectively, with high levels of syn diastereoselection. Through an assay of counterions (Rh(COD)2X), Rh(COD)2SbF6 is identified as the optimum precatalyst for reductive aldol couplings of this type. For para-substituted styryl vinyl ketones 1b-e, a progressive increase in isolated yield is observed for electron-releasing para substituents. [reaction: see text].
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
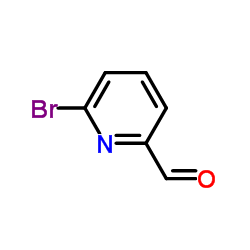 |
6-溴吡啶-2-甲醛
CAS:34160-40-2 |
C6H4BrNO |
|
Tetrathiafulvalene-based azine ligands for anion and metal c...
2015-01-01 [Beilstein J. Org. Chem. 11 , 1379-91, (2015)] |
|
Determination of amino acid enantiopurity and absolute confi...
2013-12-02 [Chemistry 19(49) , 16809-13, (2013)] |
|
First row divalent transition metal complexes of aryl-append...
2003-11-17 [Inorg. Chem. 42 , 7472-7488, (2003)] |
|
NHC-Promoted asymmetric β-lactone formation from arylal...
[J. Org. Chem. 78(8) , 3925-3938, (2013)] |
|
Synthetic methodologies leading to porphyrin-quinone conjuga...
[J. Porphyr. Phthalocyanines , 1-23, (2015)] |