The interaction of 3,5-pyrazolidinedione drugs with receptors for f-Met-Leu-Phe on human neutrophil leukocytes: a study of the structure-activity relationship.
L Levesque, R C Gaudreault, F Marceau
文献索引:Can. J. Physiol. Pharmacol. 69(3) , 419-25, (1991)
全文:HTML全文
摘要
The 3,5-pyrazolidinedione (3,5-P) drugs, phenylbutazone and sulfinpyrazone, have been reported to bind to receptors for the chemotactic peptide, f-Met-Leu-Phe, and to behave as functional antagonists of f-Met-Leu-Phe in human and rabbit neutrophils. To explore the structure-activity relationship of this family of drugs for f-Met-Leu-Phe receptor binding, 36 drugs with the 3,5-P structure, a structure related to antipyrine, or an unrelated structure were tested as competitors for the binding of f-Met-Leu-Phe-Lys-fluorescein isothiocyanate on human neutrophils by flow cytometric analysis. Only drugs possessing the 3,5-P ring were significant competitors. The five most potent 3,5-Ps behaved as selective antagonists of f-Met-Leu-Phe-induced superoxide anion release by neutrophils. The potency was not correlated to the pKa or to their capacity to inhibit prostaglandin E2 released from culture fibroblasts but instead appeared to be correlated to their apparent octanol-buffer partition coefficients. The most potent f-Met-Leu-Phe antagonist identified, 1,2-diphenyl-4-(3-(1-naphthyl)-propyl)-3,5-pyrazolidinedione (DPN), may also possess an improved pharmacodynamic specificity compared with phenylbutazone and sulfinpyrazone, as it was less potent than phenylbutazone in the inhibition of prostaglandin synthesis and it was not cytotoxic. DPN may be a prototype for a valuable new class of anti-inflammatory drugs.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
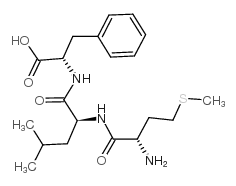 |
met-leu-phe acetate salt
CAS:59881-08-2 |
C20H31N3O4S |
|
A novel peptide-grafted liposomal delivery system targeted t...
1998-02-01 [Antimicrob. Agents Chemother. 42(2) , 348-51, (1998)] |
|
Band-selective carbonyl to aliphatic side chain 13C-13C dist...
2004-01-28 [J. Am. Chem. Soc. 126(3) , 948-58, (2004)] |
|
Arachidonic acid release in rabbit neutrophils.
1989-02-01 [Biochem. J. 257(3) , 633-7, (1989)] |
|
Alpha-1-antichymotrypsin inhibits the NADPH oxidase-enzyme c...
1992-11-01 [J. Immunol. 149(9) , 3059-65, (1992)] |
|
Effect of cytotoxic drugs on mature neutrophil function in t...
1993-06-01 [Br. J. Haematol. 84(2) , 316-21, (1993)] |