Human liver microsomal metabolism of paclitaxel and drug interactions.
P B Desai, J Z Duan, Y W Zhu, S Kouzi
文献索引:Eur. J. Drug Metab. Pharmacokinet. 23 , 417, (1998)
全文:HTML全文
摘要
The aim of this study was to investigate the influence of several anticancer drugs and investigational multidrug resistance (MDR) reversing agents on the hepatic metabolism of paclitaxel (Taxol) to its primary metabolites, 6alpha-hydroxypaclitaxel (metabolite, MA) and 3'-p-hydroxypaclitaxel (metabolite, MB). There is significant inter-individual variability associated with the levels of these two metabolites. In many cases, 6alpha-hydroxypaclitaxel has been observed to be the predominant metabolite, in others, 3'-p-hydroxypaclitaxel has been the principal metabolite. The formation of 6alpha-hydroxypaclitaxel and 3'-p-hydroxypaclitaxel is catalyzed by cytochrome P450 isozymes CYP2C8 and CYP3A4, respectively. A number of factors, including co-administration of drugs and adjuvants, are known to influence the activity of these isozymes. Therefore, the influence of MDR reversing agents, R-verapamil, cyclosporin A (CsA) and tamoxifen and anti-cancer drugs doxorubicin, etoposide (VP-16) and cisplatin on paclitaxel metabolism was assessed employing human liver microsomes in vitro. Paclitaxel (10 microM) was incubated with human liver microsomes (1 mg protein, -0.34 nmol CYP) in the presence of a NADPH generating system at 37 degrees C for 1 h, with and without the presence of interacting drug. Controls included incubations with quercetin and ketoconazole, known inhibitors of 6alpha-hydroxypaclitaxel and 3'-p-hydroxypaclitaxel formation, respectively. At the end of the incubation period, paclitaxel and the metabolites were extracted in ethyl acetate and analyzed employing an HPLC method. Significant inhibition of paclitaxel conversion to 6alpha-hydroxypaclitaxel and 3'-p-hydroxypaclitaxel was observed in the presence of R-verapamil, tamoxifen and VP-16 (P 0.005). Doxorubicin significantly inhibited the formation of 3'-p-hydroxypaclitaxel and CsA inhibited the formation of 6alpha-hydroxypaclitaxel (P 0.005). This study demonstrates that co-administration of several of the above listed compounds could lead to significant changes in the pharmacokinetics of paclitaxel.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
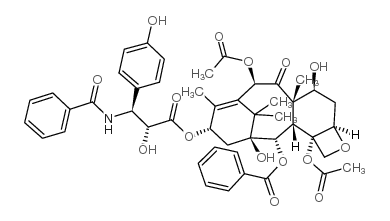 |
3'-P-HYDROXY PACLITAXEL
CAS:132160-32-8 |
C47H51NO15 |
|
Quantification of paclitaxel metabolites in human plasma by ...
1995-12-15 [J. Chromatogr. B, Biomed. Appl. 674(2) , 261-8, (1995)] |
|
Quantitative determination of paclitaxel and its metabolites...
2013-04-01 [Biomed. Chromatogr. 27(4) , 539-44, (2013)] |
|
Measurement of paclitaxel and its metabolites in human plasm...
2006-01-01 [Rapid Commun. Mass Spectrom. 20(14) , 2183-9, (2006)] |
|
Quantitation of paclitaxel and its two major metabolites usi...
2011-07-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(22) , 2018-22, (2011)] |
|
Utilization of human liver microsomes to explain individual ...
2005-01-01 [J. Pharmacol. Sci. 97(1) , 83-90, (2005)] |