Crystal structures of Escherichia coli aspartate aminotransferase in two conformations. Comparison of an unliganded open and two liganded closed forms.
J Jäger, M Moser, U Sauder, J N Jansonius
文献索引:J. Mol. Biol. 239(2) , 285-305, (1994)
全文:HTML全文
摘要
Three crystal structures of wild type E. coli aspartate aminotransferase (E.C.2.6.1.1) in space group P2(1) have been determined at resolution limits between 2.6 and 2.35 A. The unliganded enzyme and its complexes with the substrate analogues maleate and 2-methylaspartate resulted in different conformations. The unit cell parameters of the unliganded and the inhibited enzyme are a = 87.2, b = 79.9, c = 89.8 A and beta = 119.1 degrees, and a = 85.4, b = 79.8, c = 89.5 A and beta = 118.6 degrees, respectively. The crystallographic symmetry is pseudo-C222(1). The liganded enzyme structures were solved by difference Fourier techniques from that of a Val39-->Leu mutant partially refined to an R-factor of 0.22 at 2.85 A. They have a "closed" conformation like the chicken mAATase:maleate complex. The models were refined to R-factors of 0.19 (maleate complex) and 0.18 (2-methylaspartate complex) by molecular dynamics and restrained least squares methods. The unliganded crystal form was solved by molecular replacement and refined to an R-factor of 0.19 at 2.5 A resolution. The structure is in a "half-open" conformation, with the small domain rotated about 6 degrees from the closed conformation. The cofactor pyridoxal phosphate has a more relaxed conformation than in mAATase. Both maleate and 2-methylaspartate are hydrogen-bonded to the active site as in mAATase. The C alpha-CH3 bond of 2-methylaspartate is oriented at right angles to the cofactor pyridine ring, the most productive orientation for alpha-deprotonation of the substrate L-aspartate. Comparisons with earlier determined eAATase structures in space group C222(1) revealed differences that can probably be attributed to the somewhat lower resolution of the orthorhombic structures and/or mutations in the eAATases used in those studies. The present P2(1) structures confirm the justification of extrapolating properties of active site point mutants to the vertebrate isozymes. They will serve as reference in the interpretation of the properties of further site-directed mutants in continued studies of structure-function relationships of this enzyme.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
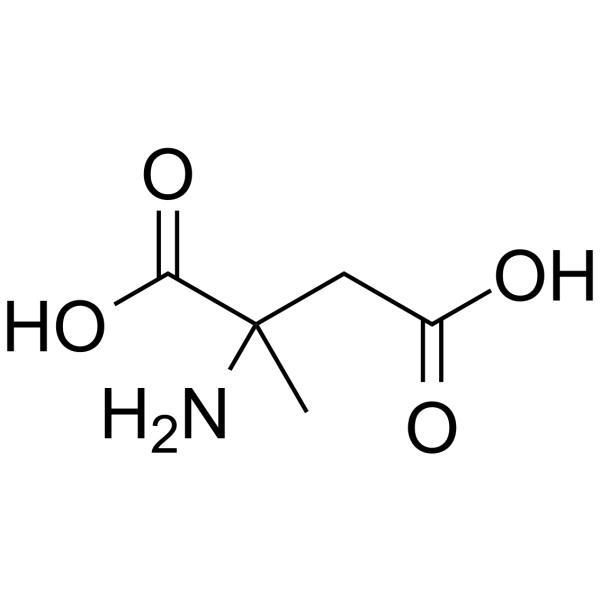 |
DL-Α-甲基天冬氨酸
CAS:2792-66-7 |
C5H9NO4 |
|
Binding of C5-dicarboxylic substrate to aspartate aminotrans...
2005-06-14 [Biochemistry 44(23) , 8218-29, (2005)] |
|
Endothelial nitric oxide production is tightly coupled to th...
2007-01-01 [Nitric Oxide 17 , 115-121, (2007)] |
|
Receptor sensitivity in bacterial chemotaxis.
2002-01-08 [Proc. Natl. Acad. Sci. U. S. A. 99(1) , 123-7, (2002)] |
|
Role of the L-citrulline/L-arginine cycle in iNANC nerve-med...
2006-09-28 [Eur. J. Pharmacol. 546(1-3) , 171-6, (2006)] |
|
Argininosuccinate synthetase is a functional target for a sn...
2009-07-24 [J. Biol. Chem. 284(30) , 20022-33, (2009)] |