Biochemistry international
1987-04-01
Order of binding of substrate to valyl-tRNA synthetase from Bacillus stearothermophilus in amino acid activation reaction.
M Kakitani, B Tonomura, K Hiromi
文献索引:Biochem. Int. 14(4) , 597-603, (1987)
全文:HTML全文
摘要
Amino acid activation reaction with valyl-tRNA synthetase (EC 6.1.1.9) from Bacillus stearothermophilus was studied kinetically by measuring ATP-PPi exchange to find the order of the binding of substrate to the enzyme. The effects of the concentration of the substrates (L-valine and ATP) and two dead-end inhibitors (L-valinol and adenosine) on the reaction rate were analyzed. The results indicate that L-valine and ATP are bound to the enzyme in a random sequence. This conclusion is consistent with the one previously suggested by static binding experiments.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
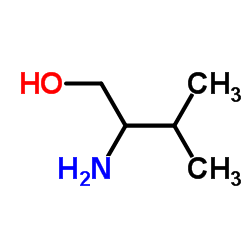 |
2-氨基-3-甲基-1-丁醇
CAS:16369-05-4 |
C5H13NO |
相关文献:
更多...
|
Chiral bis(amino alcohol)oxalamide gelators-gelation propert...
2003-11-21 [Chemistry 9(22) , 5567-80, (2003)] |
|
Synthesis of highly enantioenriched chiral alpha-aminoorgano...
2009-08-21 [J. Org. Chem. 74(16) , 5822-38, (2009)] |
|
General synthesis route to benanomicin-pradimicin antibiotic...
2007-01-01 [Chemistry 13(35) , 9791-823, (2007)] |
|
Asymmetric synthesis of 8-aminoindolizidine from chiral 2-py...
2008-11-07 [J. Org. Chem. 73(21) , 8376-81, (2008)] |
|
Dietary low-dose sucrose modulation of IQ-induced genotoxici...
2003-06-19 [Mutat. Res. 527(1-2) , 91-7, (2003)] |