N-acetylgalactosamine-6-sulfate sulfatase in human placenta: purification and characteristics.
M Masue, K Sukegawa, T Orii, T Hashimoto
文献索引:J. Biochem. 110 , 965-970, (1991)
全文:HTML全文
摘要
N-Acetylgalactosamine-6-sulfate sulfatase from human placenta was purified 33,600-fold using beta-N-acetyl-D-galactosamine 6-sulfate-(1----4)-beta-D-glucuronic acid-(1----3)-N-acetyl-D-[3H]galactosaminitol 6-sulfate as the substrate. This enzyme is an oligomer with a molecular mass of 120 kDa and consists of polypeptides of 40 and 15 kDa. The 15 kDa polypeptide is a glycoprotein. This purified protein has activities of N-acetylgalactosamine-6-sulfate sulfatase and galactose-6-sulfate sulfatase. Rabbit antiserum was raised against the purified protein. The antibody titrated N-acetylgalactosamine-6-sulfate sulfatase and galactose-6-sulfate sulfatase. The size of the precursor of the enzyme is 60 kDa, as determined by cell-free translation. The optimal pH values of the N-acetylgalactosamine-6-sulfate sulfatase and galactose-6-sulfate sulfatase activities are pH 3.8-4.0, and the Kms are 8 and 13 microM, respectively. Sulfate and phosphate ions are potent competitive inhibitors for the enzyme and their inhibition constants are 35 and 200 microM, respectively. Cross-reactive materials of 40 and 15 kDa were detected by immunoblot analysis, in the placenta, liver, and normal fibroblasts, but not in fibroblasts from a patient with Morquio disease.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
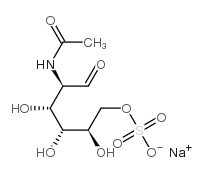 |
N-Acetyl-D-galactosamine-6-O-sulphatesodiumsalt
CAS:204575-07-5 |
C8H14NNaO9S |
|
Mucopolysaccharidosis type IVA (Morquio A disease): clinical...
2011-05-01 [Curr. Pharm. Biotechnol. 12 , 931-945, (2011)] |
|
Human N-acetylgalactosamine-4-sulphate sulphatase. Purificat...
1987-12-15 [Biochem. J. 248 , 755-764, (1987)] |
|
Human liver N-acetylgalactosamine 6-sulphatase. Purification...
1991-10-15 [Biochem. J. 279 , 515-520, (1991)] |
|
Polymorphisms in Tunisian patients with N-acetylgalactosamin...
2011-01-01 [Diagn. Pathol. 6 , 11, (2011)] |