Overexpression of biotin synthase and biotin ligase is required for efficient generation of sulfur-35 labeled biotin in E. coli.
Teegan A Delli-Bovi, Maroya D Spalding, Sean T Prigge
文献索引:BMC Biotechnol. 10 , 73, (2010)
全文:HTML全文
摘要
Biotin is an essential enzyme cofactor that acts as a CO2 carrier in carboxylation and decarboxylation reactions. The E. coli genome encodes a biosynthetic pathway that produces biotin from pimeloyl-CoA in four enzymatic steps. The final step, insertion of sulfur into desthiobiotin to form biotin, is catalyzed by the biotin synthase, BioB. A dedicated biotin ligase (BirA) catalyzes the covalent attachment of biotin to biotin-dependent enzymes. Isotopic labeling has been a valuable tool for probing the details of the biosynthetic process and assaying the activity of biotin-dependent enzymes, however there is currently no established method for 35S labeling of biotin.In this study, we produced [35S]-biotin from Na35SO4 and desthiobiotin with a specific activity of 30.7 Ci/mmol, two orders of magnitude higher than previously published methods. The biotinylation domain (PfBCCP-79) from the Plasmodium falciparum acetyl-CoA carboxylase (ACC) was expressed in E. coli as a biotinylation substrate. We found that overexpression of the E. coli biotin synthase, BioB, and biotin ligase, BirA, increased PfBCCP-79 biotinylation 160-fold over basal levels. Biotinylated PfBCCP-79 was purified by affinity chromatography, and free biotin was liberated using acid hydrolysis. We verified that we had produced radiolabeled biologically active [D]-biotin that specifically labels biotinylated proteins through reuptake in E. coli.The strategy described in our report provides a simple and effective method for the production of [35S]-biotin in E. coli based on affinity chromatography.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
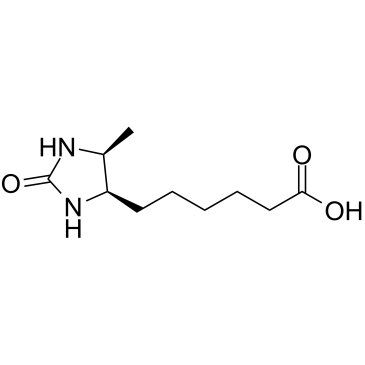 |
D-脱硫生物素
CAS:533-48-2 |
C10H18N2O3 |
|
Easily reversible desthiobiotin binding to streptavidin, avi...
2002-09-15 [Anal. Biochem. 308 , 343-357, (2002)] |
|
Direct binding of triglyceride to fat storage-inducing trans...
2011-12-06 [Proc. Natl. Acad. Sci. U. S. A. 108(49) , 19581-19586, (2011)] |
|
Mutagenesis of a flexible loop in streptavidin leads to high...
1997-08-01 [Protein Eng. 10(8) , 975-982, (1997)] |
|
Recombinant human tissue transglutaminase ELISA for the diag...
1999-12-01 [Clin. Chem. 45(12) , 2142-2149, (1999)] |
|
Fluorescence labeling of a cytokine with desthiobiotin-tagge...
2008-03-01 [J. Biosci. Bioeng. 105 , 238-242, (2008)] |