Formal total synthesis of (+/-)-estrone and zirconocene-promoted cyclization of 2-fluoro-1,7-octadienes and ru-catalyzed ring closing metathesis.
Pavel Herrmann, Milos Budesínský, Martin Kotora
文献索引:J. Org. Chem. 73(16) , 6202-6, (2008)
全文:HTML全文
摘要
A new and diastereoselective method for the synthesis of the estrone skeleton from a substituted styrene based on sequential 3-fold use of Cp 2ZrBu 2 (oxidative addition-alkylation and two cyclization-alkylation sequences) and a ruthenium complex catalyzed RC-metathesis of a sterically hindered diene was developed. The prepared estratetraene was obtained in 7 steps from a commercially available starting material and thus the overall synthesis of estrone could be accomplished in 9 steps. Moreover, we have also found that the course of the reaction of substrates bearing the 2-halo-1,7-diene moiety with Cp 2ZrBu 2, i.e., cyclization or oxidative addition to the C-X bond, could be controlled by the nature of the halogen leaving group.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
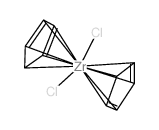 |
二氯二茂锆
CAS:1291-32-3 |
C10H2Cl2Zr |
|
Unusual temperature effects in propene polymerization using ...
2003-02-15 [ChemPhysChem 6(9) , 1929-33, (2005)] |
|
Enantioselective route from carbohydrates to cyclooctane pol...
2005-02-03 [Org. Lett. 7(3) , 511-3, (2005)] |
|
Functionalised cyclopentadienyl zirconium compounds as poten...
2008-10-21 [Dalton Trans. (39) , 5293-5, (2008)] |
|
In pursuit of pestalotiopsin a via zirconocene-mediated ring...
2006-05-25 [Org. Lett. 8(11) , 2429-31, (2006)] |
|
A selective synthesis of hydroxyborate anions as novel ancho...
2008-06-07 [Dalton Trans. (21) , 2866-70, (2008)] |