The reversible oxidation of vicinal SH groups in 4-aminobutyrate aminotransferase. Probes of conformational changes.
D S Kim, J E Churchich
文献索引:J. Biol. Chem. 262(29) , 14250-4, (1987)
全文:HTML全文
摘要
4-Aminobutyrate aminotransferase is inactivated by preincubation with iodosobenzoate at pH 7. The reaction of 2 SH residues/dimer resulted in formation of an oligomeric species of Mr = 100,000 detectable by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The subunits cross-linked via a disulfide bond are dissociated by addition of 2-mercaptoethanol which also restores full catalytic activity (Choi, S. Y., and Churchich, J.E. (1985) J. Biol. Chem. 260, 993-997). The substrate 2-oxoglutarate prevents inactivation of the enzyme by iodosobenzoate and the subsequent formation of one disulfide bond, whereas 4-aminobutyrate has no effect on the reactivity of SH groups with iodosobenzoate. Modified 4-aminobutyrate aminotransferase (containing 1 disulfide bond) catalyzes a half-transamination reaction; but it is unable to react with 2-oxoglutarate to generate the aldimine form of the enzyme. The spectroscopic properties (fluorescence yield and polarization of fluorescence) of PMP bound to the modified enzyme are different from those of pyridoxamine phosphate (PMP) bound to the native enzyme. The polarization of fluorescence values of PMP bound to the cross-linked enzyme, excited over the spectral range 310-370 nm, are greater (25%) than those of the cofactor of the native enzyme. An increase in the polarization values implies that the motion of PMP is restricted when the subunits are cross-linked via a disulfide bond.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
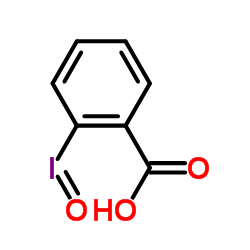 |
2-亚碘酰苯甲酸
CAS:304-91-6 |
C7H5IO3 |
|
Reduction of nitroaromatic compounds by anaerobic bacteria i...
1991-04-01 [Appl. Environ. Microbiol. 57(4) , 962-8, (1991)] |
|
Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphata...
1987-08-25 [J. Biol. Chem. 262(24) , 11714-20, (1987)] |
|
Epitopes of Escherichia coli ribosomal protein S13.
1989-12-01 [J. Protein Chem. 8(6) , 701-17, (1989)] |
|
Influence of the polymer-micelle interaction on micelle-subs...
2010-03-01 [J. Pharm. Sci. 99(3) , 1440-51, (2010)] |
|
Tryptic digestion of bovine secretory IgA at elevated temper...
1989-01-01 [Int. J. Biochem. 21(5) , 549-54, (1989)] |