Phenothiazine-binding and attachment sites of CAPP1-calmodulin.
D L Newton, C B Klee
文献索引:Biochemistry 28(9) , 3750-7, (1989)
全文:HTML全文
摘要
In the presence of Ca2+ norchlorpromazine isothiocyanate forms a monocovalent complex with calmodulin: CAPP1-calmodulin (Newton et al, 1983). Trypsin digestion of [3H]CAPP1-calmodulin yields as the major radioactive peptide N epsilon-CAPP-Lys-Met-Lys, corresponding to residues 75-77 of calmodulin. Stoichiometric amounts of all other expected tryptic peptides are also found, indicating that norchlorpromazine isothiocyanate selectively acylates Lys 75. A second molecule of CAPP-NCS can react, albeit slowly, with calmodulin to form CAPP2-calmodulin. Fragments 38-74 and 127-148 are completely missing from the trypsin digests of CAPP2-calmodulin without deliberate exposure to UV irradiation. Possibly the lengthy preparation of CAPP2-calmodulin favors photolysis, caused by room lights, of the putative CAPP-binding domains located in these two peptides. Lys 148, the sole lysyl residue in fragment 127-148, is a probable site of attachment of the second molecule of CAPP. UV irradiation of CAPP1-calmodulin, followed by digestion with trypsin, results in the selective loss of 50% each of peptides containing residues 38-74 and 127-148, suggesting that these peptides contain the hydrophobic amino acids that form the phenothiazine-binding sites. The loss of peptides encompassing residues 38-74 and 127-148, located in the amino and carboxyl halves of calmodulin, respectively, suggests that the hydrophobic rings of CAPP can bind at either one of the two phenothiazine sites. Computer modeling of CAPP1-calmodulin with the X-ray coordinates of calmodulin (Babu et al., 1986) indicates that CAPP attached to Lys 75 cannot interact with the carboxyl-terminal phenothiazine-binding site.(ABSTRACT TRUNCATED AT 250 WORDS)
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
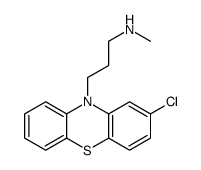 |
3-(2-chlorophenothiazin-10-yl)-N-methylpropan-1-amine
CAS:1225-64-5 |
C16H17ClN2S |
|
Prediction of drug responses in schizophrenia: a method usin...
1997-08-01 [Psychiatry Clin. Neurosci. 51(4) , 217-22, (1997)] |
|
Concentrations of chlorpromazine and two of its active metab...
1981-01-01 [Psychopharmacology 73(1) , 55-62, (1981)] |
|
[A microanalytical gas liquid chromatography method with a n...
1984-09-30 [Boll. Soc. Ital. Biol. Sper. 60(9) , 1757-63, (1984)] |
|
[N-dealkylation of chlorimipramine and chlorpromazine in pat...
1984-09-30 [Boll. Soc. Ital. Biol. Sper. 60(9) , 1727-33, (1984)] |
|
Relationships between drug concentrations in serum and CSF, ...
1984-01-01 [Acta Psychiatr. Scand. Suppl. 311 , 49-74, (1984)] |