Synthesis of optically pure highly functionalized gamma-lactams via 2-azetidinone-tethered iminophosphoranes.
Benito Alcaide, Pedro Almendros, Jose M Alonso
文献索引:J. Org. Chem. 69(3) , 993-6, (2004)
全文:HTML全文
摘要
A synthesis of optically pure densely functionalized gamma-lactams starting from 2-azetidinone-tethered iminophosphoranes has been developed. Full chirality transfer has been accomplished from the enantiomerically pure 2-azetidinones. The addition of lithium acetylides to 4-oxoazetidine-2-carbaldehydes at -78 degrees C smoothly yielded propargylic alcohols with excellent diastereoselectivities. Propargylic alcohols were converted to mesylates, which by exposure to sodium azide afforded the corresponding azides. Treatment of beta-lactams bearing an azido side chain with triphenylphosphine (TPP) gave lambda(5)-phosphazenes (iminophosphoranes, phosphine imines), which were not isolated. The sodium methoxide promoted reaction of the phosphazene beta-lactams smoothly provided gamma-lactams, through a N1-C2 bond breakage process on the four-membered lactam with concomitant ring expansion, followed by hydrolysis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
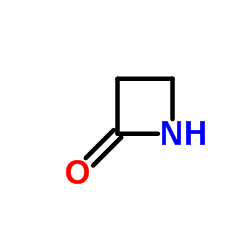 |
2-氮杂环丁酮
CAS:930-21-2 |
C3H5NO |
|
The influence of ezetimibe on classical and alternative acti...
2014-08-01 [Naunyn Schmiedebergs Arch. Pharmacol. 387(8) , 733-42, (2014)] |
|
Non-transpeptidase binding arylthioether β-lactams active ag...
2015-02-01 [Bioorg. Med. Chem. 23(3) , 632-47, (2015)] |
|
Photoelectron spectra of some antibiotic building blocks: 2-...
2012-08-23 [J. Phys. Chem. A 116(33) , 8653-60, (2012)] |
|
2-Azetidinone (ß-propiolactam) Holley RW and Holley AD.
[J. Am. Chem. Soc. 71(6) , 2129-31, (1949)] |