Catalytic asymmetric bromination and chlorination of beta-ketoesters.
Mauro Marigo, Nagaswami Kumaragurubaran, Karl Anker Jørgensen
文献索引:Chemistry 10 , 2133-2137, (2004)
全文:HTML全文
摘要
The first general catalytic asymmetric bromination and chlorination of beta-ketoesters has been developed. The reactions proceed for both acyclic and cyclic beta-ketoesters catalyzed by chiral bisoxazolinecopper(II) complexes giving the corresponding optically active alpha-bromo- and alpha-chloro-beta-ketoesters in high yields and moderate to good enantioselectivities. For the optically active chlorinated products the isolated yields are in the range of 88-99 % and the enantiomeric excesses up to 77 % ee, while the optically active brominated adducts are formed in 70-99 % isolated yield and up to 82 % ee. Based on the absolute configuration of the optically active products, the face selectivity for the catalytic enantioselective halogenation is discussed based on a bidentate coordination of the beta-ketoester to the chiral catalyst and a X-ray structure of chiral alpha,gamma-diketoesterenolatebisoxazolinecopper(II) complex.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
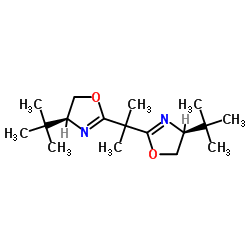 |
(S,S)-(-)-2,2'-异丙叉双(4-特丁基-2-噁唑啉)
CAS:131833-93-7 |
C17H30N2O2 |
|
Direct catalytic asymmetric aldol reactions of pyruvates: sc...
2004-04-07 [Org. Biomol. Chem. 2 , 1077-1085, (2004)] |
|
Protection of neonatal mice from group B streptococcal infec...
1992-12-01 [Tetrahedron 60 , 4989-4994, (2004)] |
|
Gant, T.G. Meyers, A.I.
[Tetrahedron 50 , 2297, (1994)] |
|
Pfaltz, A.
[Acc. Chem. Res. 26 , 339, (1993)] |
|
Bolm, C.
[Angew. Chem. Int. Ed. Engl. 30 , 542, (1991)] |