Excitatory amino acid agonists. Enzymic resolution, X-ray structure, and enantioselective activities of (R)- and (S)-bromohomoibotenic acid.
J J Hansen, B Nielsen, P Krogsgaard-Larsen, L Brehm, E O Nielsen, D R Curtis
文献索引:J. Med. Chem. 32(10) , 2254-60, (1989)
全文:HTML全文
摘要
The enantiomers of alpha-amino-4-bromo-3-hydroxy-5-isoxazolepropionic acid (4-bromohomoibotenic acid, Br-HIBO, 1) a selective and potent agonist at one class of the central (S)-glutamic acid receptors, were prepared with an enantiomeric excess higher than 98.8% via stereoselective enzymic hydrolysis of (RS)-alpha-(acetylamino)-4-bromo-3-methoxy-5-isoxazolepropionic acid (4) using immobilized aminoacylase. The absolute configuration of the enantiomers of Br-HIBO was established by X-ray crystallographic analysis, which confirmed the expected preference of the enzyme for the S form of the substrate 4. (S)- and (RS)-Br-HIBO were potent neuroexcitants on cat spinal neurones in vivo, while (R)-Br-HIBO was a very weak excitant. Correspondingly, the S enantiomer of Br-HIBO (IC50 = 0.34 microM) was considerably more potent than the R form (IC50 = 32 microM) as an inhibitor of [3H]-(RS)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ([ 3H]AMPA) binding to rat brain synaptic membranes in vitro. In contrast, (S)- and (R)-Br-HIBO were approximately equipotent (IC50 values of 0.22 and 0.15 microM, respectively) as inhibitors of [3H]-(S)-glutamic acid binding in the presence of CaCl2. The enantiomers of Br-HIBO showed no significant affinity for those binding sites on rat brain membranes which are labeled by [3H]kainic acid or [3H]-(R)-aspartic acid.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
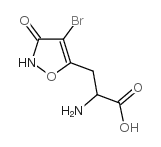 |
(Rs)-4-溴高鹅膏蕈氨酸
CAS:71366-32-0 |
C6H7BrN2O4 |
|
Turning behaviour and catalepsy after injection of excitator...
1981-05-29 [Neurosci. Lett. 23 , 337, (1981)] |
|
Structural determinants of agonist-specific kinetics at the ...
2005-08-23 [Proc. Natl. Acad. Sci. U. S. A. 102(34) , 12053-8, (2005)] |
|
Identification of amino acid residues in GluR1 responsible f...
2001-05-01 [J. Neurosci. 21(9) , 3052-62, (2001)] |
|
4-Methylhomoibotenic acid activates a novel metabotropic glu...
1997-11-01 [J. Pharmacol. Exp. Ther. 283(2) , 742-9, (1997)] |
|
4-Bromohomoibotenic acid selectively activates a 1-aminocycl...
1994-07-01 [J. Neurochem. 63(1) , 133-9, (1994)] |