Melanogenesis inhibition by tetrahydropterines.
F García-Molina, J L Munoz-Munoz, J R Acosta, P A García-Ruiz, J Tudela, F García-Cánovas, J N Rodríguez-López
文献索引:Biochim. Biophys. Acta 1794 , 1766-1774, (2009)
全文:HTML全文
摘要
There is controversy in the literature concerning the action of tetrahydropterines on the enzyme tyrosinase and on melanogenesis in general. In this study, we demonstrate that tetrahydropterines can inhibit melanogenesis in several ways: i) by non-enzymatic inhibition involving purely chemical reactions reducing o-dopaquinone to L-dopa, ii) by acting as substrates which compete with L-tyr and L-dopa, since they are substrates of tyrosinase; and iii) by irreversibly inhibiting the enzymatic forms met-tyrosinase and deoxy-tyrosinase in anaerobic conditions. Three tetrahydropterines have been kinetically characterised as tyrosinase substrates: 6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, 6-methyl-5,6,7,8-tetrahydropterine and 6,7-(R,S)-dimethyl-5,6,7,8-tetrahydropterine. A kinetic reaction mechanism is proposed to explain the oxidation of these compounds by tyrosinase.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
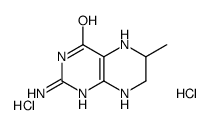 |
(±)-6-甲基-5,6,7,8-四氢蝶啶二盐酸盐
CAS:69113-63-9 |
C7H13Cl2N5O |
|
Simple assay procedure for tyrosine hydroxylase activity by ...
[J. Chromatogr. A. 427 , 229, (1988)] |
|
A tyrosine hydroxylase assay in microwells using coupled non...
1991-01-01 [Anal. Biochem. 192 , 125, (1991)] |
|
Analytical characterization of a sensitive radioassay for ty...
2004-08-01 [Neurochem. Res. 29 , 1467-1472, (2004)] |
|
A unique dual activity amino acid hydroxylase in Toxoplasma ...
2009-01-01 [PLoS ONE 4 , e4801, (2009)] |