Cleavage and deprotection of peptide resins using chloro- and bromotrialkylsilanes.
J L Hughes, E J Leopold
文献索引:Pept. Res. 8(5) , 298-300, (1995)
全文:HTML全文
摘要
The cleavage of a peptide resin attached to a phenylacetomidomethyl (PAM) resin was investigated using bromotrimethylsilane (TMSBr) with thioanisole in trifluoroacetic acid (TFA), and by chlorotrimethylsilane (TMSCl) in the same reagents with lithium bromide. Both TMSBr and TMSCl cleaved the peptide from the resin, but TMSCl required elevated temperature (50 degrees C) to effect the cleavage. Procedures were investigated for the deprotection and cleavage of either N(g)-tosyl (Tos)- or mesitylenesulfonyl (MTS)-arginine residues attached to 4-methylbenzylhydrylamine (MBHA) resin, and of a peptide containing an MTS-arginine residue attached to MBHA resin, using either TMSCl or TMSBr as cleavage reagents. The MTS group is cleanly removed from arginine using TMSBr with thioanisole in TFA and by TMSCl in the same reagents with lithium bromide.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
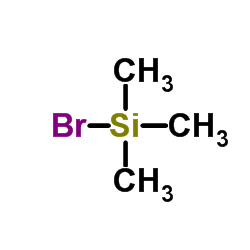 |
三甲基溴硅烷
CAS:2857-97-8 |
C3H9BrSi |
|
EPR and NMR spectroscopies provide input on the coordination...
2015-06-01 [J. Biol. Inorg. Chem. 20 , 719-27, (2015)] |
|
Position and length of fatty acids strongly affect receptor ...
2014-11-01 [ChemMedChem 9(11) , 2463-74, (2014)] |
|
Reduced vascular endothelial growth factor and capillary den...
2014-07-01 [Brain Pathol. 24(4) , 334-43, (2014)] |
|
Two-step hard acid deprotection/cleavage procedure for solid...
1991-02-01 [Int. J. Pept. Protein Res. 37(2) , 145-52, (1991)] |
|
The chemoselective and efficient deprotection of silyl ether...
2008-06-21 [Org. Biomol. Chem. 6(12) , 2168-72, (2008)] |