The aspartimide problem in Fmoc-based SPPS. Part I.
M Mergler, F Dick, B Sax, P Weiler, T Vorherr
文献索引:J. Pept. Sci. 9 , 36-46, (2003)
全文:HTML全文
摘要
A variety of Asp beta-carboxy protecting groups, Hmb backbone protection and a range of Fmoc cleavage protocols have been employed in syntheses of the model hexapeptide H-VKDGYI-OH to investigate the aspartimide problem in more detail. The extent of formation of aspartimide and aspartimide-related by-products was determined by RP-HPLC. This study included three new Fmoc-Asp-OH derivatives: the beta-(4-pyridyl-diphenylmethyl) and beta-(9-phenyl-fluoren-9-yl) esters and also the orthoester Fmoc-beta-(4-methyl-2,6,7-trioxabicyclo[2.2.2]-oct-1-yl)-alanine. 3-Methylpent-3-yl protection of the Asp side chain resulted in significant improvements with respect to aspartimide formation. Complete suppression was achieved using the combination OtBu side chain protection and Hmb backbone protection for the preceding Gly residue.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
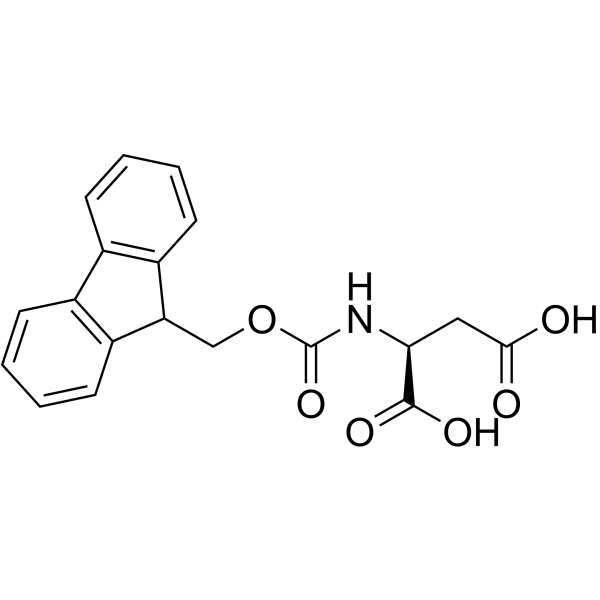 |
Fmoc-L-天冬氨酸(DOD)
CAS:119062-05-4 |
C19H17NO6 |
|
Synthesis of N-linked glycopeptides via solid-phase aspartyl...
2010-08-21 [Org. Biomol. Chem. 8 , 3723-3733, (2010)] |
|
The aspartimide problem in Fmoc-based SPPS. Part II.
2003-08-01 [J. Pept. Sci. 9 , 518-526, (2003)] |
|
The aspartimide problem in Fmoc-based SPPS. Part III.
2005-10-01 [J. Pept. Sci. 11 , 650-657, (2005)] |
|
Synthesis and CD structural studies of CD52 peptides and gly...
2008-11-24 [Carbohydr. Res. 343 , 2894-2902, (2008)] |
|
On-resin convergent synthesis of a glycopeptide from HIV gp1...
2011-01-01 [Methods Mol. Biol. 751 , 343-355, (2011)] |