Hyperexpression of recombinant CFTR in heterologous cells alters its physiological properties.
R Mohammad-Panah, S Demolombe, D Riochet, V Leblais, G Loussouarn, H Pollard, I Baró, D Escande
文献索引:Am. J. Physiol. 274(2 Pt 1) , C310-8, (1998)
全文:HTML全文
摘要
We investigated whether high levels of expression of the cystic fibrosis transmembrane conductance regulator (CFTR) would alter the functional properties of newly synthesized recombinant proteins. COS-7, CFPAC-1, and A549 cells were intranuclearly injected with a Simian virus 40-driven pECE-CFTR plasmid and assayed for halide permeability using the 6-methoxy-N-(3-sulfopropyl)quinolinium fluorescent probe. With increasing numbers of microinjected pECE-CFTR copies, the baseline permeability to halide dose dependently increased, and the response to adenosine 3',5'-cyclic monophosphate (cAMP) stimulation decreased. In cells hyperexpressing CFTR, the high level of halide permeability was reduced when a cell metabolism poisoning cocktail was applied to decrease intracellular ATP and, inversely, was increased by orthovanadate. In CFPAC-1 cells investigated with the patch-clamp technique, CFTR hyperexpression led to a time-independent nonrectifying chloride current that was not sensitive to cAMP stimulation. CFPAC-1 cells hyperexpressing CFTR exhibited no outward rectifying chloride current nor inward rectifying potassium current either spontaneously or under cAMP stimulation. We conclude that hyperexpression of recombinant CFTR proteins modifies their properties inasmuch as 1) CFTR channels are permanently activated and not susceptible to cAMP regulation and 2) they lose their capacity to regulate heterologous ionic channels.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
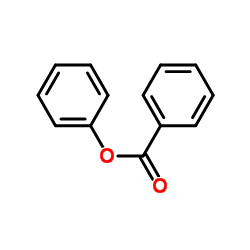 |
苯甲酸苯酯
CAS:93-99-2 |
C13H10O2 |
|
Pressurized liquid extraction-gas chromatography-mass spectr...
2016-04-01 [J. Chromatogr. A. 1440 , 37-44, (2016)] |
|
Discrimination of skin sensitizers from non-sensitizers by i...
2016-09-01 [J. Appl. Toxicol. 36 , 1129-36, (2016)] |
|
Chloride current activated by cyclic AMP and parathyroid hor...
1989-10-01 [Pflugers Arch. 415(1) , 104-14, (1989)] |
|
Cellular ATP release by the cystic fibrosis transmembrane co...
1996-02-01 [Am. J. Physiol. 270(2 Pt 1) , C538-45, (1996)] |
|
Potassium efflux from infant and adult rat choroid plexuses:...
1994-03-14 [Neurosci. Lett. 169(1-2) , 207-11, (1994)] |