Experimental and molecular dynamics simulation study of the sublimation energetics of cyclopentadienyltricarbonylmanganese (Cymantrene).
Ricardo Picciochi, José N Canongia Lopes, Hermínio P Diogo, Manuel E Minas da Piedade
文献索引:J. Phys. Chem. A 112(41) , 10429-34, (2008)
全文:HTML全文
摘要
The standard molar enthalpy of sublimation of monoclinic cyclopentadienyltricarbonylmanganese, Mn(eta (5)-C 5H 5)(CO) 3, at 298.15 K, was determined as Delta sub H m (o)[Mn(eta (5)-C 5H 5)(CO) 3] = 75.97 +/- 0.37 kJ x mol (-1) from Knudsen effusion and Calvet-drop microcalorimetry measurements, thus considerably improving the very large inaccuracy (>10 kJ x mol (-1)) of the published data. The obtained value was used to assess the extension of the OPLS-based all-atom force field we previously developed for iron metallocenes to manganese organometallic compounds. The modified force field was able to reproduce the volumetric properties (density and unit-cell volume) of crystalline Mn(eta (5)-C 5H 5)(CO) 3 with a deviation of 0.6% and the experimentally determined enthalpy of sublimation with an accuracy of 1 kJ x mol (-1). The interaction (epsilon) and atomic-diameter (sigma) parameters of the Lennard-Jones (12-6) potential function used to calculate dispersion contributions within the framework of the force field were found to be transferable from iron to manganese.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
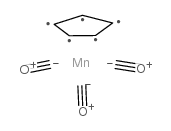 |
三羰基环戊二烯合锰
CAS:12079-65-1 |
C8H5MnO3 |
|
Synthesis, characterization, X-ray crystallography, and cyto...
2008-12-01 [J. Inorg. Biochem. 102(12) , 2114-9, (2008)] |
|
Potentiality of an organometallic labelled streptavidin--bio...
1991-01-01 [J. Pharm. Biomed. Anal. 9(10-12) , 965-7, (1991)] |
|
Pulmonary activation and toxicity of cyclopentadienyl mangan...
1996-02-01 [Toxicol. Appl. Pharmacol. 136(2) , 280-8, (1996)] |
|
Occupational and environmental exposure of garage workers an...
1994-01-01 [Am. Ind. Hyg. Assoc. J. 55(1) , 53-8, (1994)] |
|
Synthesis and biological activity of cymantrene and cyrhetre...
2012-06-07 [Dalton Trans. 41(21) , 6443-50, (2012)] |