The importance of the hydroxyl moieties for inhibition of the Ca(2+)-ATPase by trilobolide and 2,5-di(tert-butyl)-1,4-benzohydroquinone.
M Wictome, M Holub, J M East, A G Lee
文献索引:Biochem. Biophys. Res. Commun. 199(2) , 916-21, (1994)
全文:HTML全文
摘要
Trilobolide and 2,5-di(tert-butyl)-1,4-benzohydroquinone (BHQ) are potent inhibitors of the Ca(2+)-ATPase of skeletal muscle sarcoplasmic reticulum. Desoxytrilobolide and 2,5-di(tert-butyl)-1,4-diacetylphenol (acetyl-BHQ) have much lower potencies than their parent compounds and 2,5-di(tert-butyl)-1,4-benzoquinone (BQ) has no effect on ATPase activity. Studies using the ATPase labelled with 4-nitrobenzo-2-oxa-1,3-diazole (NBD) suggest that both trilobolide and BHQ bind more strongly to the E2 conformation of the ATPase than to the E1 conformation. Desoxytrilobolide, acetyl-BHQ and BQ have little effect on the E1/E2 equilibrium. Studies with mixtures of trilobolide and desoxytrilobolide suggest that the inactive derivatives are unable to bind to the ATPase.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
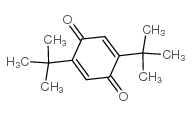 |
2,5-二叔丁基-1,4-苯醌
CAS:2460-77-7 |
C14H20O2 |
|
P2u purinoceptor modulation of intracellular Ca2+ in a human...
1996-10-01 [Cell Calcium 20(4) , 339-46, (1996)] |
|
Redox proteins as electron acceptors from chlorophyll triple...
1990-10-01 [Photochem. Photobiol. 52(4) , 883-91, (1990)] |
|
Preclinical evaluation and molecular docking of 2,5-di-tert-...
2015-01-01 [Int. J. Bioinform. Res. Appl. 11(2) , 142-52, (2015)] |
|
Pressure effects on cation migration in 2, 5-di-tert-butyl-1...
[Int. J. Chem. Kinet. 33(7) , 397-401, (2001)] |
|
Calcium oscillations in parotid acinar cells induced by micr...
1992-03-01 [Am. J. Physiol. 262(3 Pt 1) , C656-63, (1992)] |