Analysis of the beta-endorphin structure-related activity on human monocyte chemotaxis: importance of the N- and C-terminal.
P Sacerdote, A E Panerai
文献索引:Peptides 10 , 565-569, (1989)
全文:HTML全文
摘要
We evaluated the chemotactic activity of beta-endorphin and beta-endorphin-related peptides on human monocytes. We tested beta-endorphin(1-31) and fragments (1-16), (1-17), (1-27) in which the N-terminal of the opioid is preserved, N-acetyl-beta-endorphin(1-31) and fragments (6-31) and (28-31) in which the C-terminal is preserved, and fragment (2-17) that lacks both the N- and C-terminal. The fragments in which the N- and C-terminal were preserved [with the exception of fragment (28-31)] showed a chemotactic effect, while the lack of both terminals deprived the peptides of any activity. Moreover, only the N-terminal-mediated effects were naloxone reversible, while the C-terminal effects were not. These results indicate that while the intact N-terminal is necessary for opioid like effects, both N- and C-terminal can mediate effects on the immune system, thus offering evidence for a nonopioid receptor-mediated effect of opioid peptides on the immune system.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
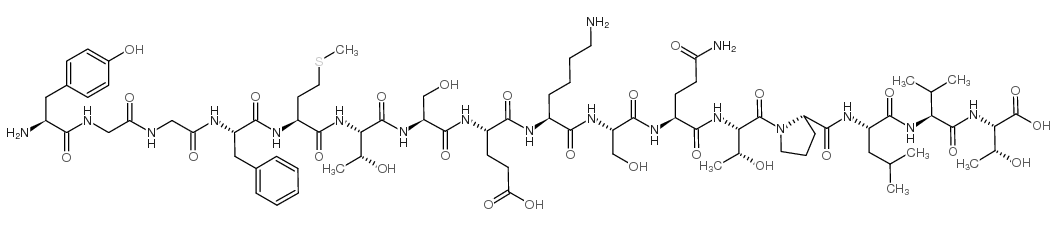 |
α-内啡肽
CAS:59004-96-5 |
C77H120N18O26S |
|
Live cell monitoring of mu-opioid receptor-mediated G-protei...
2007-09-14 [J. Biol. Chem. 282 , 27126-32, (2007)] |
|
gamma endorphin, alpha endorphin and Met-enkephalin are form...
1977-10-13 [Nature 269(5629) , 619-21, (1977)] |
|
Selective conversion of beta-endorphin into peptides related...
1980-01-03 [Nature 283(5742) , 96-7, (1980)] |
|
Effects of [Des-Tyr 1]-gamma-endorphin and alpha-endorphin o...
1979-06-01 [Pharmacol. Biochem. Behav. 10(6) , 899-905, (1979)] |
|
Isolation, primary structure, and synthesis of alpha-endorph...
1976-11-01 [Proc. Natl. Acad. Sci. U. S. A. 73(11) , 3942-6, (1976)] |