Mn(III)-Based Oxidative Free-Radical Cyclizations of Unsaturated Ketones.
Bridget McCarthyCole, Luning Han, BarryB. Snider
文献索引:J. Org. Chem. 61(22) , 7832-7847, (1996)
全文:HTML全文
摘要
Mn(III)-based oxidative free-radical cyclization of unsaturated ketones is a versatile synthetic procedure with broad applicability. For example, oxidation of cyclopentanone 1a with 2 equiv of Mn(OAc)(3).2H(2)O and 1 equiv of Cu(OAc)(2).H(2)O in AcOH at 80 degrees C for 1.5 h affords 75% of bicyclo[3.2.1]oct-3-en-8-one 8a and 15% of bicyclo[3.2.1]oct-2-en-8-one 9a. Bridged bicyclic ketones that cannot enolize further are isolated in good yield. Monocyclic beta,gamma-unsaturated ketones that can enolize are oxidized further to give gamma-acetoxy enones. The formation of bicyclo[3.3.1]non-2-en-9-one (57a) in 52% yield from 2-allylcyclohexanone (56a) suggests that kinetically controlled enolization is the rate-determining step in alpha-keto radical formation. A wide variety of examples delineating the scope, limitations, and stereoselectivity of this reaction are presented.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
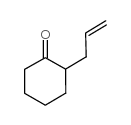 |
2-烯丙基环己酮
CAS:94-66-6 |
C9H14O |
|
A simplified procedure for epoxidation by benzonitrile-hydro...
[Tetrahedron 18(6) , 763-65, (1962)] |
|
Stereochemical Studies. IX. Asymmetric Synthesis of 2-Alkylc...
[Chem. Pharm. Bull. 20 , 246-57, (1972)] |
|
A Modified Thermodynamically Controlled Deracemization of 2-...
[Chem. Lett. 33(5) , 516-17, (2004)] |
|
Biphase and triphase catalysis. Arsonated polystyrenes as ca...
[J. Am. Chem. Soc. 101(23) , 6938-46, (1979)] |