Purification and characterization of organic solvent stable protease from Bacillus licheniformis RSP-09-37.
Ritu Sareen, Prashant Mishra
文献索引:Appl. Microbiol. Biotechnol. 79 , 399-405, (2008)
全文:HTML全文
摘要
A protease was purified from the cell-free supernatant of Bacillus licheniformis RSP-09-37, a mutant from a thermophilic bacterial strain, B. licheniformis RSP-09, using affinity chromatography with alpha-casein agarose resin. The protease was purified 85-fold to electrophoretic homogeneity. The apparent molecular mass of purified protease was 55 kDa using gel filtration in high-performance liquid chromatography, which is in agreement with the results obtained from sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggesting a monomeric nature of the protein. The purified protease revealed temperature optima of 50 degrees C and pH optima of 10.0 and was classified as serine protease based on its complete inhibition with phenyl methyl sulfonyl fluoride. The purified protease exhibited tolerance to both detergents and organic solvent. The synthetic activity of the protease was tested using the transesterification reaction between N-acetyl-L-phenylalanine-ethyl ester and n-propanol in organic solvents varying in their log P values and the kinetic parameters of the enzyme in these organic solvents were studied. The enzyme has potential to be employed for synthetic reactions and in detergent formulations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
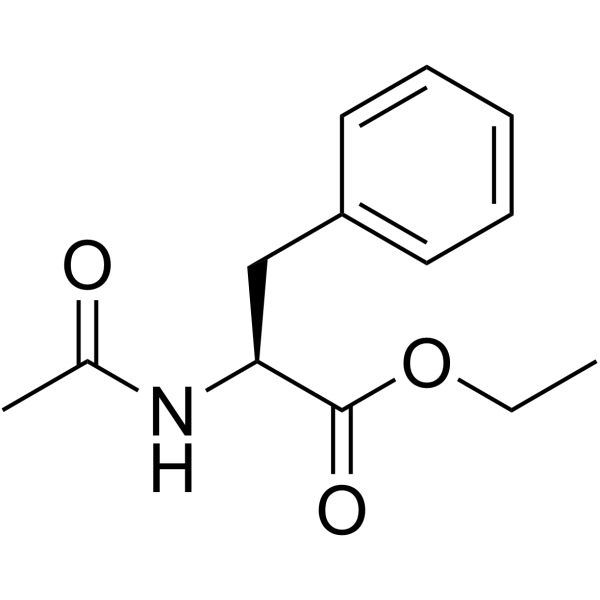 |
N-乙酰-L-苯丙氨酸乙酯
CAS:2361-96-8 |
C13H17NO3 |
|
Surface charge fine tuning of reversed-phase/weak anion-exch...
2015-08-28 [J. Chromatogr. A. 1409 , 189-200, (2015)] |
|
The effect of enzymatic reaction on dissolution rate: theore...
1986-02-01 [J. Pharm. Sci. 75(2) , 195-203, (1986)] |
|
Immobilization of enzymes on fumed silica nanoparticles for ...
2011-01-01 [Methods Mol. Biol. 743 , 147-160, (2011)] |
|
Protease activation in glycerol-based deep eutectic solvents...
2011-12-01 [J. Mol. Catal., B Enzym. 72 , 163-167, (2011)] |
|
High transesterification activities of immobilized proteases...
2010-08-01 [Biotechnol. Lett. 32 , 1109-1116, (2010)] |