Development of new N-Arylbenzamides as STAT3 Dimerization Inhibitors.
MuraliK Urlam, Roberta Pireddu, Yiyu Ge, Xiaolei Zhang, Ying Sun, HarshaniR Lawrence, WayneC Guida, Sa_dM Sebti, NicholasJ Lawrence
文献索引:MedChemComm 4(6) , 932-941, (2013)
全文:HTML全文
摘要
The O-tosylsalicylamide S3I-201 (10) was used as a starting point for design and synthesis of novel STAT-3 dimerization inhibitors with improved drug-like qualities. The phosphonic acid 12d and salicylic acids 13f, 13g with a shorter amide linker lacking the O-tosyl group had improved STAT-3 inhibitory activity. The equivalent potencies observed by the replacement of phosphonic acid moiety of 12d with 5-amino-2-hydroxybenzoic acid group as in 13f further validates 5-amino-2-hydroxybenzoic acid as a phosphotyrosine mimic. The salicylic acid 13f displayed improved whole cell activity. The focused library of salicylic acids 13 with benzamide linker indicated that hydrophobic heptyl and cyclohexyl are the best tolerated R groups and a biphenyl ether (as the Ar group) significantly contributes to STAT3 inhibitory activity. Our docking studies indicated that the acidic groups of 12d, 13f and 13g interact in the p-Tyr-705 binding site in a broadly similar manner, while the phenoxybenzoyl group and the cyclohexylbenzyl group occupying pY+1 and pY-X hydrophobic pockets respectively. The in vitro and cell based potency of 13f warrants further development of this scaffold as STAT3 inhibitors.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
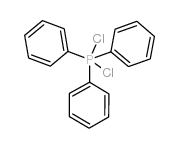 |
三苯基二氯化膦
CAS:2526-64-9 |
C18H15Cl2P |
|
Simple and convenient synthesis of tertiary benzanilides usi...
[Tetrahedron 59(13) , 2325-31, (2003)] |
|
Cleavage of carboxylic acid esters to acid chlorides with di...
[J. Org. Chem. 40(21) , 3026-3032., (1975)] |
|
Synthesis of N-Phosphopeptides Coupled by Dichlorotriphenylp...
[Synth. Commun. 31(13) , 2067-75, (2001)] |
|
The preparation of dimethyl phosphorochloridite and its homo...
[Phosph. Sulfur Relat. Elem. 33(1-2) , 37-39., (1987)] |
|
Synthesis, crystal structure and dynamic behavior of naphtha...
[J. Mol. Struct. 891(1) , 346-350., (2008)] |