Reduction of benzyl halides by liver microsomes. Formation of 478 NM-absorbing sigma-alkyl-ferric cytochrome P-450 complexes.
D Mansuy, M Fontecave
文献索引:Biochem. Pharmacol. 32(12) , 1871-9, (1983)
全文:HTML全文
摘要
The benzyl halides benzyl bromide and 4-nitrobenzyl chloride are reduced anaerobically by NADPH and rat liver microsomes to yield toluene and 4-nitrotoluene, respectively. These reductions and cytochrome P-450-dependent since they are inhibited by CO and metyrapone, and are increased after pretreatment of rats by phenobarbital and 3-methylcholanthrene. During benzyl halide reduction, cytochrome P-450 complexes, which are very unstable to O2 and characterized by a Soret peak at 478 nm, are formed in steady-state concentrations. These concentrations are very dependent on pretreatment of rats and on the nature of the reducing agent (NADPH or dithionite) and the benzyl halide:4-methylbenzyl bromide and benzyl bromide lead to 478 nm absorbing complexes in the presence of NADPH whereas 4-nitrobenzyl chloride and benzyl chloride lead to such completes only in the presence of dithionite. Microsomal reductions of 4-nitrobenzyl chloride and benzyl bromide in D2O lead to partially deuterated 4-nitrotoluene and toluene. From these results, we propose a mechanism for anaerobic microsomal reduction of benzyl halides involving the intermediate formation of sigma-alkyl cytochrome P-450-Fe(III)-CH2Ar complexes which exhibit red-shifted Soret peaks around 478 nm. Toluenes, ArCH3, are formed either by protonation of the sigma-alkyl complexes or by hydrogen abstraction by the intermediate free radical ArCH2.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
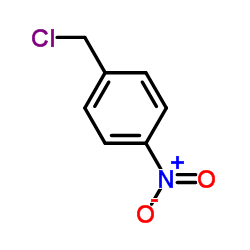 |
4-硝基氯化苄
CAS:100-14-1 |
C7H6ClNO2 |
|
A heme model system for the reduction of substrates by micro...
1982-02-26 [Biochem. Biophys. Res. Commun. 104(4) , 1651-7, (1982)] |
|
A case of multiple sensitization to three halogeno-substitut...
1978-01-01 [Ital. Gen. Rev. Dermatol. 15(2) , 107-12, (1978)] |
|
Hepatic and branchial glutathione S-transferases of two fish...
2009-05-01 [Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 149(4) , 515-23, (2009)] |
|
Reactivity, SCE induction and mutagenicity of benzyl chlorid...
1983-08-01 [J. Appl. Toxicol. 3(4) , 203-7, (1983)] |
|
Evaluation of hepatic glutathione transferase Mu 1 and Theta...
2012-03-01 [Drug Metab. Dispos. 40(3) , 497-503, (2012)] |