Kinetic characterization of recombinant human glutathione transferase T1-1, a polymorphic detoxication enzyme.
P Jemth, B Mannervik
文献索引:Arch. Biochem. Biophys. 348(2) , 247-54, (1997)
全文:HTML全文
摘要
Recombinant human theta class glutathione transferase T1-1 has been heterologously expressed in Escherichia coli and a simple purification method involving immobilized ferric ion affinity chromatography and Orange A dye chromatography is described. The catalytic properties of the enzyme differ significantly from those of other glutathione transferases, also within the theta class, with respect to both substrate selectivity and kinetic parameters. In addition to 1,2-epoxy-3-(4-nitrophenoxy)propane, the substrate used previously to monitor the enzyme, human glutathione transferase T1-1 has activity with the naturally occurring phenethylisothiocyanate and also displays glutathione peroxidase activity with cumene hydroperoxide. Further, the enzyme is active with 4-nitrobenzyl chloride and 4-nitrophenethyl bromide, but shows no detectable activity with the more chemically reactive 1-chloro-2,4-dinitrobenzene. The Michaelis constant for glutathione, K(m)GSH, with 1,2-epoxy-3-(4-nitrophenoxy)propane as second substrate, is high at low pH values but decreases at higher pH values. This is mirrored in kcat/K(m)GSH which increases with an apparent pKa value of 9.0, reflecting the ionization of the thiol group of glutathione in solution. The same results are obtained with 4-nitrophenethyl bromide as electrophilic substrate, although the K(m)GSH value (0.72 mM at pH 7.5), as well as the pKa (8.1) derived from the pH dependence of kcat/K(m)GSH, are lower with this substrate. In contrast, kcat and kcat/K(m)electrophile display either a maximum or a plateau at pH 7.0-7.5, and an apparent pKa value of 5.7 was determined for the pH dependence of kcat with both 4-nitrophenethyl bromide and 1,2-epoxy-3-(4-nitrophenoxy)propane as electrophilic substrates. This pKa value reflects an ionization of enzyme-bound GSH, most probably involving the sulfhydryl group, whose pKa value thus is lowered by the enzyme. Three differences in the cDNA as compared to the sequence previously published were found. One of these differences causes a change in the deduced amino acid sequence and involves the nucleotide triplet encoding amino acid 126, which was determined as GAG (Glu), instead of the published GGG (Gly).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
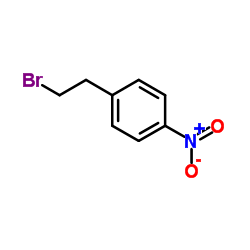 |
4-硝基苯乙基溴
CAS:5339-26-4 |
C8H8BrNO2 |
|
An ensemble of theta class glutathione transferases with nov...
2002-04-19 [J. Mol. Biol. 318(1) , 59-70, (2002)] |
|
Residue 234 in glutathione transferase T1-1 plays a pivotal ...
2005-05-15 [Biochem. J. 388(Pt 1) , 387-92, (2005)] |
|
A glutathione S-transferase (GST) isozyme from broccoli with...
1994-03-16 [Biochim. Biophys. Acta 1205(1) , 29-38, (1994)] |
|
Enzymatic inactivation of major circulating forms of atrial ...
1999-04-16 [Eur. J. Pharmacol. 370(3) , 307-12, (1999)] |
|
Molecular evolution of Theta-class glutathione transferase f...
2010-05-01 [Arch. Biochem. Biophys. 497(1-2) , 28-34, (2010)] |