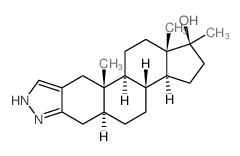
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
BV8077000
-
CHEMICAL NAME :
-
1'H-Androstano(3,2-c)pyrazol-17-ol, 17-methyl-, (5-alpha,17-beta)-
-
CAS REGISTRY NUMBER :
-
302-96-5
-
LAST UPDATED :
-
199103
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C21-H32-N2-O
-
MOLECULAR WEIGHT :
-
328.55
-
WISWESSER LINE NOTATION :
-
T G5 E6 B665 HNN HUTJ M1 Q1 RQ R1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
24 mg/kg/17W-I
-
TOXIC EFFECTS :
-
Liver - jaundice, other or unclassified
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 294,612,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
4285 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Liver - jaundice, other or unclassified
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 294,612,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,375,1982 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4800 ug/kg
-
SEX/DURATION :
-
female 13-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 4,2088,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
female 13-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - homeostasis
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 4,2088,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
14 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 5,489,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
36 mg/kg
-
SEX/DURATION :
-
female 12 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
REFERENCE :
-
CPBTAL Chemical and Pharmaceutical Bulletin. (Japan Pub. Trading Co., USA, 1255 Howard St., San Francisco, CA 94103) V.6- 1958- Volume(issue)/page/year: 14,285,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1100 ug/kg
-
SEX/DURATION :
-
male 2 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - other effects on male Endocrine - androgenic
-
REFERENCE :
-
THGNBO Theriogenology. (Geron-X, Inc., P.O. Box 1108, Los Altos, CA 94022) V.1- 1974- Volume(issue)/page/year: 28,109,1987
|