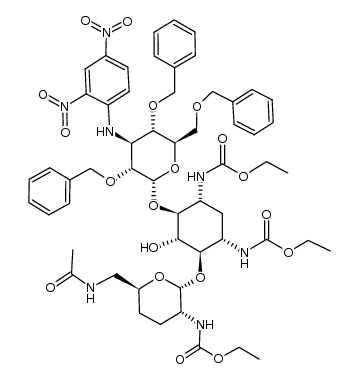
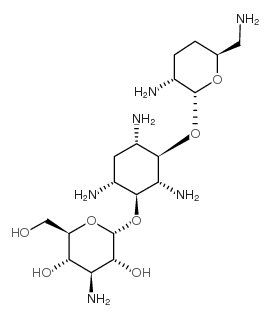
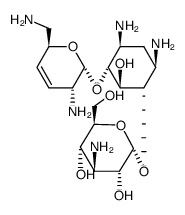
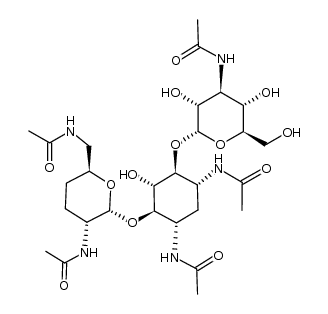
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WK1985000
-
CHEMICAL NAME :
-
D-Streptamine, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1-6)-O-(2,6 -diamino-2,3,4,6- tetradeoxy-alpha-D-erythro-hexopyranosyl)-(1-4)-2-deo xy-
-
CAS REGISTRY NUMBER :
-
34493-98-6
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C18-H37-N5-O8
-
MOLECULAR WEIGHT :
-
451.60
-
WISWESSER LINE NOTATION :
-
T6OTJ CZ F1Z BO- BL6TJ AZ CQ EZ DO- BT6OTJ CQ DZ EQ F1Q
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>6950 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,221,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
16760 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
23870 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
12510 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
763 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
11960 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
15980 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
8950 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 6,119,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
373 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JOPHDQ Journal of Pharmacobio-Dynamics. (Japan Pub. Trading Co. (USA), 1255 Howard St., San Francisco, CA 94103) V.1- 1978- Volume(issue)/page/year: 4,356,1981 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
750 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Kidney, Ureter, Bladder - changes in bladder weight Related to Chronic Data - changes in testicular weight
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 20,7481,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3900 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Kidney, Ureter, Bladder - changes in bladder weight Related to Chronic Data - death
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 20,7541,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
31500 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Ear) - changes in vestibular functions Sense Organs and Special Senses (Ear) - changes in cochlear structure or function Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
APTOA6 Acta Pharmacologica et Toxicologica. (Copenhagen, Denmark) V.1-59, 1945-86. For publisher information, see PHTOEH Volume(issue)/page/year: 53,230,1983 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 10-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 8-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
3600 mg/kg
-
SEX/DURATION :
-
female 8-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 8-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 8-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 8-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 26,40,1973
|

![(4R,6S,7R,7aS)-7-(((2R,3R,6S)-6-(acetamidomethyl)-3-(((benzyloxy)carbonyl)amino)tetrahydro-2H-pyran-2-yl)oxy)-6-(((benzyloxy)carbonyl)amino)-2,2-dimethyl-4-nitrohexahydrobenzo[d][1,3]dioxol-5-yl acetate结构式](https://image.chemsrc.com/caspic/427/113886-52-5.png)