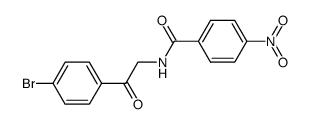
5-(4-溴苯基)-2-(4-硝基苯基)噁唑
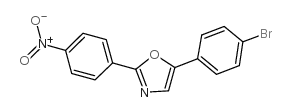
5-(4-溴苯基)-2-(4-硝基苯基)噁唑结构式

|
常用名 | 5-(4-溴苯基)-2-(4-硝基苯基)噁唑 | 英文名 | 5-(4-BROMO-PHENYL)-2-(4-NITRO-PHENYL)-OXAZOLE |
|---|---|---|---|---|
| CAS号 | 118426-04-3 | 分子量 | 345.14800 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C15H9BrN2O3 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
| 中文名 | 5-(4-溴苯基)-2-(4-硝基苯基)噁唑 |
|---|---|
| 英文名 | 5-(4-bromophenyl)-2-(4-nitrophenyl)-1,3-oxazole |
| 英文别名 | 更多 |
| 分子式 | C15H9BrN2O3 |
|---|---|
| 分子量 | 345.14800 |
| 精确质量 | 343.98000 |
| PSA | 71.85000 |
| LogP | 5.20250 |
| InChIKey | HQFYSZAPVRHQNX-UHFFFAOYSA-N |
| SMILES | O=[N+]([O-])c1ccc(-c2ncc(-c3ccc(Br)cc3)o2)cc1 |
|
~90% 
5-(4-溴苯基)-2-(4-... 118426-04-3 |
| 文献:Shvaika, O. P.; Korzhenevskaya, N. G.; Snagoshchenko, L. P. Chemistry of Heterocyclic Compounds (New York, NY, United States), 1985 , vol. 21, # 2 p. 157 - 160 Khimiya Geterotsiklicheskikh Soedinenii, 1985 , vol. 21, # 2 p. 193 - 197 |
|
~31% 
5-(4-溴苯基)-2-(4-... 118426-04-3 |
| 文献:Haldon, Estela; Besora, Maria; Cano, Israel; Cambeiro, Xacobe C.; Pericas, Miquel A.; Maseras, Feliu; Nicasio, M. Carmen; Perez, Pedro J. Chemistry - A European Journal, 2014 , vol. 20, # 12 p. 3463 - 3474 |
|
~% 
5-(4-溴苯基)-2-(4-... 118426-04-3 |
| 文献:BRISTOL-MYERS SQUIBB COMPANY; LOPEZ, Omar D.; ST. LAURENT, Denis R.; GOODRICH, Jason; ROMINE, Jeffrey Lee; SERRANO-WU, Michael; YANG, Fukang; KAKARLA, Ramesh; YANG, Xuejie; QIU, Yuping; SNYDER, Lawrence B. Patent: WO2011/82077 A1, 2011 ; |
|
~% 
5-(4-溴苯基)-2-(4-... 118426-04-3 |
| 文献:BRISTOL-MYERS SQUIBB COMPANY; LOPEZ, Omar D.; ST. LAURENT, Denis R.; GOODRICH, Jason; ROMINE, Jeffrey Lee; SERRANO-WU, Michael; YANG, Fukang; KAKARLA, Ramesh; YANG, Xuejie; QIU, Yuping; SNYDER, Lawrence B. Patent: WO2011/82077 A1, 2011 ; |
|
实验名称:Primary cell-based high-throughput screening assay for identification of compounds th...
来源:Johns Hopkins Ion Channel Center
靶标:regulator of G-protein signaling 4 isoform 2 [Homo sapiens]
External Id:JHICC_RGS_Act_HTS
|
|
实验名称:Luminescence-based cell-based primary high throughput screening assay to identify ago...
来源:The Scripps Research Institute Molecular Screening Center
靶标:mu-type opioid receptor isoform MOR-1 [Homo sapiens]
External Id:OPRM1-OPRD1_AG_LUMI_1536_1X%ACT PRUN
|
|
实验名称:QFRET-based biochemical primary high throughput screening assay to identify exosite i...
来源:The Scripps Research Institute Molecular Screening Center
靶标:disintegrin and metalloproteinase domain-containing protein 17 preproprotein [Homo sapiens]
External Id:ADAM17_INH_QFRET_1536_1X%INH PRUN
|
|
实验名称:Fluorescence-based cell-based primary high throughput screening assay to identify ago...
来源:The Scripps Research Institute Molecular Screening Center
靶标:muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id:CHRM1_AG_FLUO8_1536_1X%ACT PRUN
|
|
实验名称:uHTS identification of small molecule activators of the adaptive arm of the Unfolded ...
来源:Burnham Center for Chemical Genomics
靶标:N/A
External Id:BCCG-A405-UPR-XBP1-PrimaryAgonist-Assay
|
|
实验名称:Antibacterial activity against Klebsiella pneumoniae MDR ATCC 70063 (CO-ADD:GN_003); ...
来源:ChEMBL
靶标:Klebsiella pneumoniae
External Id:CHEMBL4296186
|
|
实验名称:High throughput fluorescence intensity-based biochemical assay to screen for small mo...
来源:University of Pittsburgh Molecular Library Screening Center
靶标:furin (paired basic amino acid cleaving enzyme), isoform CRA_a [Homo sapiens]
External Id:MH080376 Biochemical HTS for Inhibitors of the Proprotein Convertase Furin.
|
|
实验名称:Fluorescence polarization to screen for inhibitor that competite the binding of FadD2...
来源:Broad Institute
靶标:FATTY-ACID-CoA LIGASE FADD28 (FATTY-ACID-CoA SYNTHETASE)
External Id:2147-01_Inhibitor_SinglePoint_HTS_Activity
|
|
实验名称:Bursicon-induced LGR2 mediated cAMP production in LGR-2/CRE6x-Luciferase co-transfect...
来源:Broad Institute
靶标:N/A
External Id:Bursicon-induced LGR2 mediated cAMP production in LGR-2/CRE6x-Luciferase co-transfected HEK293 cells Inhibition - 7011-01_Antagonist_SinglePoint_HTS_Activity
|
|
实验名称:Identifying Sarm1 TIR NADase inhibitors through high throughput HPLC assay
来源:24386
靶标:N/A
External Id:Sarm1 TIR NADase inhibitors
|
| 2-(4-nitrophenyl)-5-(4-bromophenyl)oxazole |
| 5-(4-bromophenyl)-2-(4-nitrophenyl)oxazole |