Ethyl 3-oxo-3-phenylpropanoate
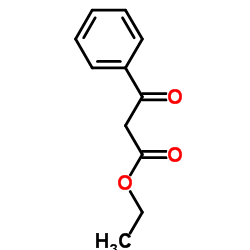
Ethyl 3-oxo-3-phenylpropanoate structure
|
Common Name | Ethyl 3-oxo-3-phenylpropanoate | ||
|---|---|---|---|---|
| CAS Number | 94-02-0 | Molecular Weight | 192.211 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 268.1±13.0 °C at 760 mmHg | |
| Molecular Formula | C11H12O3 | Melting Point | < 0ºC | |
| MSDS | Chinese USA | Flash Point | 113.1±19.9 °C | |
|
A practical and convenient fluorination of 1,3-dicarbonyl compounds using aqueous HF in the presence of iodosylbenzene.
Org. Lett. 13 , 2392-2394, (2011) A simple, practical, and convenient fluorination of 1,3-dicarbonyl compounds was achieved by direct use of aqueous hydrofluoric acid and iodosylbenzene (PhIO). The reaction of ethyl benzoylacetate with the reagent system of aqueous HF and PhIO in CH(2)Cl(2) g... |
|
|
Reaction of iodonium ylides of 1,3-dicarbonyl compounds with HF reagents.
Molecules 17(6) , 6625-32, (2012) Reaction of dibenzoylmethane with (diacetoxyiodo)benzene in the presence of KOH in MeCN quantitatively gave the corresponding iodonium ylide, which was treated with a HF reagent to afford the corresponding 2-fluorinated dibenzoylmethane in 14-50% yields. The ... |
|
|
A short synthesis of the triazolopyrimidine antibiotic essramycin.
J. Nat. Prod. 73(11) , 1938-9, (2010) A short synthesis of the 1,2,4-triazolo[1,5-a]pyrimidine antibiotic essramycin is described involving condensation of aminoguanidine with ethyl benzoylacetate to give an amino-1,2,4-triazole, followed by condensation with ethyl acetoacetate to form the pyrimi... |
|
|
Free radical reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds.
Org. Biomol. Chem. 7(19) , 4074-81, (2009) A manganese(III)-mediated reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds that produces benzo[c]furan-4,7-diones and anthracene-1,4-diones with high chemoselectivity is described. With ethyl butyrylacetate, by changing the solvent, b... |
|
|
3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno [2,3-d]pyrimidine: an effecient key precursor for novel synthesis of some interesting triazines and triazepines as potential anti-tumor agents.
Molecules 17(10) , 11538-53, (2012) A number of interesting heterocycles were prepared through interaction of the intermediate 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno-[2,3-d]pyrimidine (1) and reagents such as hydrazonyl halides 2 to furnish triazine derivatives 4a-l... |
|
|
Asymmetric synthesis of both enantiomers of fluoxetine via microbiological reduction of ethyl benzoylacetate. Chênevert R, et al.
Tetrahedron 48(33) , 6769-76, (1992)
|
|
|
The reaction of ethyl benzoylacetate with malononitrile: a novel synthesis of some pyridazine, pyridazino [2, 3-a] quinazoline and pyrrole derivatives. Abdelrazek FM, et al.
Tetrahedron 57(9) , 1813-17, (2001)
|