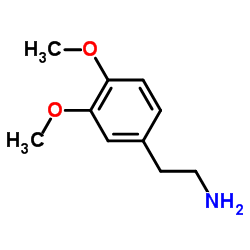
|
~92% |
|
~% |
|
~% |
|
~% |
|
~93% |
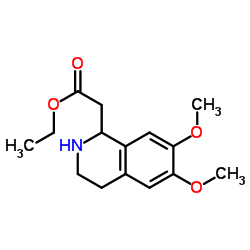
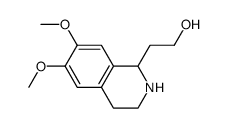
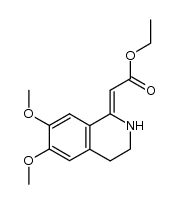
![Propanoic acid,3-[[2-(3,4-dimethoxyphenyl)ethyl]amino]-3-oxo-,ethyl ester Structure](https://image.chemsrc.com/caspic/069/79641-41-1.png)