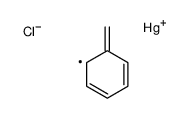
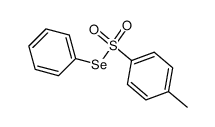
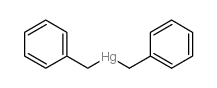
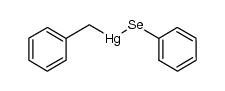
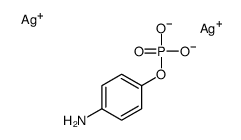
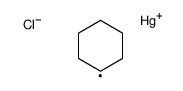
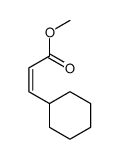
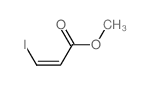
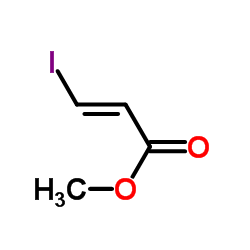
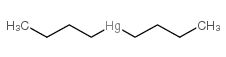
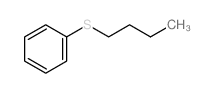
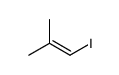
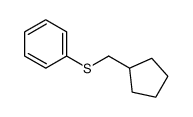
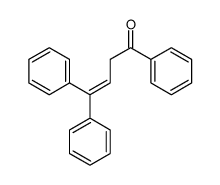
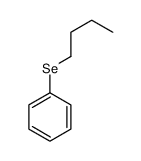
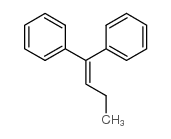
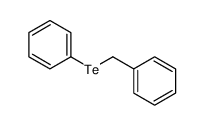
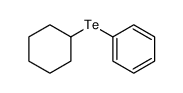
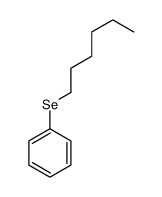
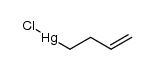
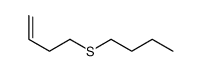
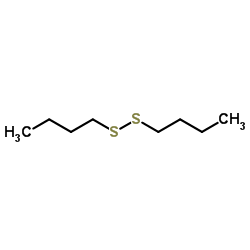
|
~83% |
|
~96% |
|
~98% |
|
~93% |
|
~93% |
|
~% |
|
~99% |
|
~99% |
|
~44% |
|
~58% |
|
~% |
|
~72% |
|
~72% |
|
~87% |
|
~87% |
|
~88% |
|
~27% |
|
~86% |
|
~% |
|
~45% |
|
~% |
|
~54% |
|
~53% |
|
~69% |
|
~77% |
|
~68% |
|
~% |
|
~% |
|
~66% |
|
~65% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~99% |
|
~10% |
|
~% |
|
~99% |
|
~99% |
|
~46% |
|
~47% |
|
~87% |
|
~73% |
|
~65% |
|
~92% |
|
~92% |
|
~56% |
|
~10% |
|
~10% |
|
~% |
|
~97% |
|
~0% |
|
~98% |
|
~80% |
|
~71% |
|
~69% |
|
~83% |
|
~79% |
|
~79% |
|
~61% |
|
~61% |
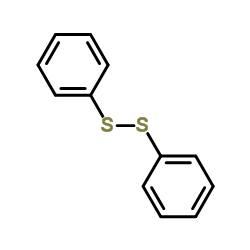
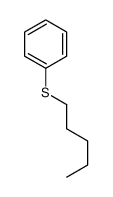
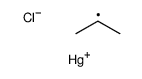
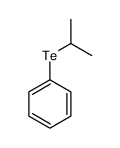



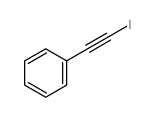
![Benzene,[(2-phenylethynyl)sulfonyl] Structure](https://image.chemsrc.com/caspic/359/5324-64-1.png)
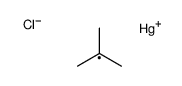
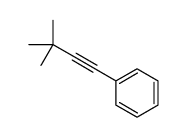


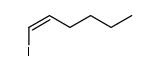
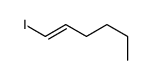
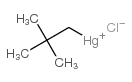
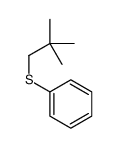
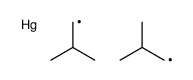
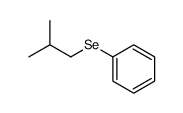
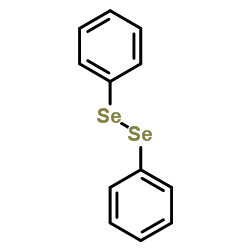
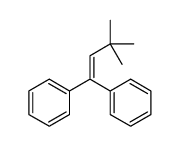

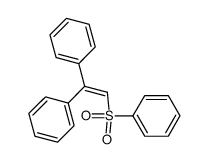

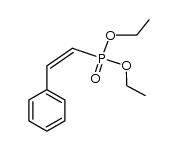
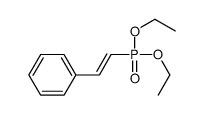
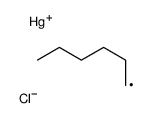

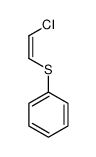
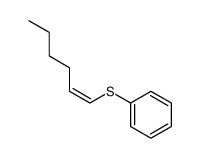
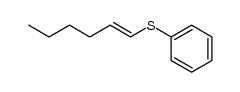
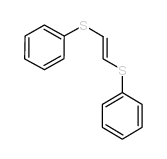
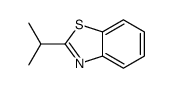
![2-Iodobenzo[d]thiazole Structure](https://image.chemsrc.com/caspic/276/1123-99-5.png)