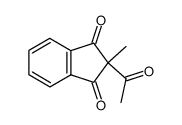
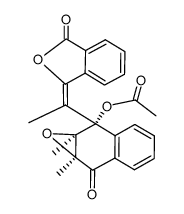
|
~16% |
|
~21% |
|
~15% |
|
~%
Detail
|
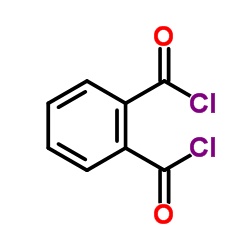
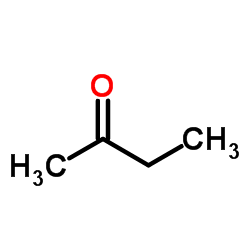
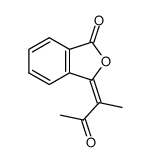
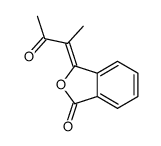
![1a,7a-dimethylnaphtho[2,3-b]oxirene-2,7-dione Structure](https://image.chemsrc.com/caspic/258/53948-58-6.png)