Recent advances in radical-based C–N bond formation via photo-/electrochemistry
Yating Zhao, Wujiong Xia
Index: 10.1039/C7CS00572E
Full Text: HTML
Abstract
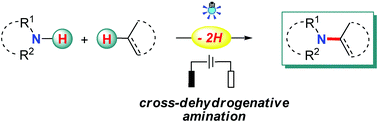
The employment of nitrogen sources with free N–H bonds for amination is considered to be most straightforward and desirable, especially when the C–N bonds are prepared from N–H bonds and non-functionalized carbon sources, such as C–H bonds and C–C double/triple bonds, since this obviates the needs for the pre-installation of reactive groups in the starting materials and leads to a high atom and step economy. Recently, radical chemistry has been resuscitated owing to its great value in organic synthesis, and notable advances have been made in the direct use of N–H bonds for radical-based C–N bond formation with photo-/electrotechniques. Apart from the well-studied N-radical species addition pathway, radical-mediated aminations also proceed through N-atom nucleophilic addition, C-/N-radical cross-coupling, and a hydrogen-atom transfer (HAT) process. This review highlights the recent advances in this area with emphasis on the related reaction mechanisms.
|
Structure-based design of targeted covalent inhibitors
2018-04-05 [10.1039/C7CS00220C] |
|
Correction: Spotting the differences in two-dimensional mate...
2018-04-04 [10.1039/C8CS90042F] |
|
Wearable and flexible electronics for continuous molecular m...
2018-04-03 [10.1039/C7CS00730B] |
|
Multimetallic nanosheets: synthesis and applications in fuel...
2018-04-03 [10.1039/C8CS00113H] |
|
Liquid metals: fundamentals and applications in chemistry
2018-04-03 [10.1039/C7CS00043J] |