RSC Advances
2018-04-09
An optimized procedure for direct access to 1H-indazole-3-carboxaldehyde derivatives by nitrosation of indoles
Arnaud Chevalier, Abdelaaziz Ouahrouch, Alexandre Arnaud, Thibault Gallavardin, Xavier Franck
Index: 10.1039/C8RA01546E
Full Text: HTML
Abstract
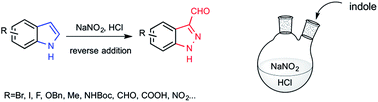
Indazole derivatives are currently drawing more and more attention in medicinal chemistry as kinase inhibitors. 1H-indazole-3-carboxaldehydes are key intermediates to access to a variety of polyfunctionalized 3-substituted indazoles. We report here a general access to this motif, based on the nitrosation of indoles in a slightly acidic environment. These very mild conditions allow the conversion of both electron-rich and electron-deficient indoles into 1H-indazole-3-carboxaldehydes.
Latest Articles:
More...
|
Thermoelectric properties of polycrystalline palladium sulfi...
2018-04-09 [10.1039/C8RA01613E] |
|
The effect of sintering process on lithium ionic conductivit...
2018-04-09 [10.1039/C8RA01329B] |
|
Magnetic surface molecularly imprinted poly(3-aminophenylbor...
2018-04-09 [10.1039/C8RA01250D] |
|
Weavable asymmetric carbon nanotube yarn supercapacitor for ...
2018-04-09 [10.1039/C8RA01384E] |
|
Synergistic regulation mechanism of iperoxo and LY2119620 fo...
2018-04-09 [10.1039/C8RA01545G] |