An Efficient Synthesis of exo/endo‐Hydroxylated Cyclohexenones by Thiol/Amine‐Mediated Tandem Aldol–[2,3]‐Sigmatropic Rearrangement: Amine‐Dependent Complementary Regioselectivity
Motofumi Miura; Toshinori Nakakita; Masaharu Toriyama; Shigeyasu Motohashi
Index: 10.1002/ajoc.201800081
Full Text: HTML
Abstract
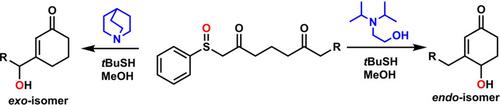
exo/endo‐Hydroxylated cyclohexenones are essential synthetic intermediates for a variety of biologically active compounds. However, methods that lead to efficient and selective preparation of exo/endo‐hydroxylated cyclohexenones remain to be established. Herein, we report the practical and scalable synthesis of exo/endo‐hydroxylated cyclohexenones from readily available 2,6‐diketosulfoxides. We demonstrate that endo and exo geometric isomers of hydroxylated cyclohexenones can be obtained by simple modification of the reaction conditions.
|
A Copper‐Catalyzed Domino Reaction of Alkynyl Bromides and O...
2018-04-14 [10.1002/ajoc.201800154] |
|
Sequential Ytterbium(III) Triflate Catalyzed One‐Pot Three‐C...
2018-04-10 [10.1002/ajoc.201800087] |
|
Recent Progress in Using Pyrene‐4,5‐diketones and Pyrene‐4,5...
2018-04-10 [10.1002/ajoc.201800039] |
|
Chelation‐assisted β‐selective direct C‐H bond arylation of ...
2018-04-06 [10.1002/ajoc.201800160] |
|
Radical Reaction for the Synthesis of Thiopyrans under Photo...
2018-04-06 [10.1002/ajoc.201800159] |