Asymmetric Organocatalysis and Photoredox Catalysis for the α-Functionalization of Tetrahydroisoquinolines
Hong Hou, Shaoqun Zhu, Iuliana Atodiresei, Magnus Rueping
Index: 10.1002/ejoc.201800117
Full Text: HTML
Abstract
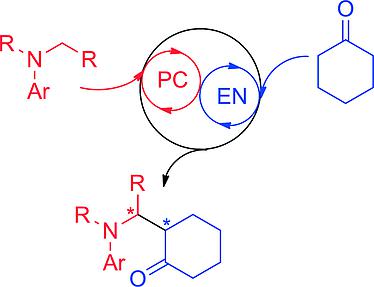
The asymmetric α-alkylation of tetrahydroisoquinolines with cyclic ketones has been accomplished in the presence of a combined catalytic system consisting of a visible-light photoredox catalyst and a chiral primary amine organocatalyst. The desired products were obtained in good yields, high enantioselectivity, and good to excellent diastereoselectivity. (PC: photoredox cycle, EN: enamine cycle). A combined photoredox/organocatalytic system for the asymmetric α-alkylation of tetrahydroisoquinolines (THIQs) has been developed. In this protocol, a chiral organocatalyst enables a high level of enantio- and diastereocontrol for the oxidative coupling reaction of THIQs with simple cyclic ketones.
|
Development of Photoactivatable Nitroxyl (HNO) Donors Incorp...
2018-04-15 [10.1002/ejoc.201800092] |
|
Catalytic C‐Alkylation of Pyrroles with Primary Alcohols: Ha...
2018-03-30 [10.1002/ejoc.201800146] |
|
Fluorine‐Containing Functionalized Cyclopentene Scaffolds Th...
2018-03-30 [10.1002/ejoc.201800057] |
|
Verdazyl Radical Building Blocks: Synthesis, Structure, and ...
2018-03-30 [10.1002/ejoc.201701783] |
|
Iron‐Catalyzed Sulfur‐Promoted Decyanative Redox Condensatio...
2018-03-30 [10.1002/ejoc.201701607] |