An efficient synthesis of multisubstituted 4-nitrobuta-1,3-dien-1-amines and application in cyclisation reactions
Brigita Vigante, Aiva Plotniece, Martins Rucins, Marina Petrova, Ruslan Muhamadejev, Karlis Pajuste, Sergey Belyakov, Yuriy G. Shermolovich, Arkadij Sobolev
Index: 10.1016/j.tet.2018.03.075
Full Text: HTML
Abstract
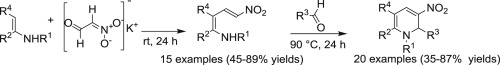
The synthesis of multisubstituted 4-nitrobuta-1,3-dien-1-amines (nitrodienamines) from 3-aminocrotonates and nitroacetaldehyde potassium salt, has been performed in 45–89% yields. This one-step protocol works efficiently with a broad range of N-H and N-substituted 3-aminocrotonates and delivers both primary and secondary nitrodienamines. In addition, the possible variations of the substituents at the positions 2 and 3 of 4-nitrobuta-1,3-dien-1-amine have been shown. Generally, the yields of secondary 4-nitrobuta-1,3-dien-1-amines were lower than those of primary ones. The synthetic usefulness of obtained 4-nitrobuta-1,3-dien-1-amines has also been demonstrated by achieving the synthesis of multisubstituted 5-nitro-1,6-dihydropyridines in two-component cyclocondensation reactions of 4-nitrobuta-1,3-dien-1-amines with aromatic or aliphatic aldehydes. Lastly, diverse N-H and N-substituted 5-nitro-1,6-dihydropyridines have been obtained in 35–87% yields.
|
Synthesis of chiral α-amino anilides via a DMEDA-promoted se...
2018-04-11 [10.1016/j.tet.2018.03.003] |
|
A site isolation-enabled organocatalytic approach to enantio...
2018-04-11 [10.1016/j.tet.2018.04.022] |
|
Chemoselectivity in the Kosugi-Migita-Stille coupling of bro...
2018-04-10 [10.1016/j.tet.2018.02.051] |
|
Process design methodology for organometallic chemistry in c...
2018-04-07 [10.1016/j.tet.2018.04.020] |
|
Substrate engineering: Effects of different N-protecting gro...
2018-04-07 [10.1016/j.tet.2018.04.012] |