Pd-Induced Double C–H Bond Activation in Annulative Syntheses of Bipyrrole Boomerangs: Mechanistic Insights from NMR Spectroscopy and Computation
Marika Żyła-Karwowska, Liliia Moshniaha, Halina Zhylitskaya, Marcin Stępień
Index: 10.1021/acs.joc.8b00630
Full Text: HTML
Abstract
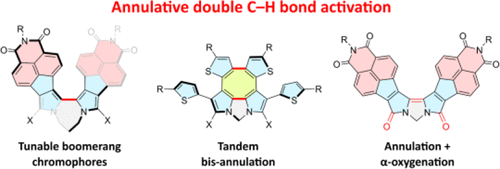
1,n-Dipyrrolylalkanes can be efficiently converted into extensively π-conjugated bipyrroles by PdII-mediated annulative double C–H activation, and this approach might be further developed into tandem processes involving further cyclization of substituents or oxygenation of pyrrolic α-positions. Herein, the mechanism of these transformations is explored using NMR spectroscopy and DFT calculations. The kinetics of the annulation are found to depend on the conjugation extent and donor–acceptor character of the pyrroles, as well as on substitution and the linker length. Combined experimental and theoretical evidence indicates that a change of the rate-determining step occurs for the most electron-deficient substrates. The unprecedented double α-oxygenation of bipyrroles is found to be a stepwise process, involving α-acetoxylated intermediates.
|
Synthesis and Unique Optical Properties of Selenophenyl BODI...
2018-04-19 [10.1021/acs.joc.8b00782] |
|
Synthesis of 3,5-Disubstituted BODIPYs Bearing N-Containing ...
2018-04-17 [10.1021/acs.joc.8b00087] |
|
Steric Hindrance Underestimated: It is a Long, Long Way to T...
2018-04-17 [10.1021/acs.joc.8b00496] |
|
Stereoselective Sequential [4+2]/[2+2] Cycloadditions Involv...
2018-04-16 [10.1021/acs.joc.8b00346] |
|
DiCE: Diastereomeric in Silico Chiral Elucidation, Expanded ...
2018-04-16 [10.1021/acs.joc.8b00338] |