Two Temperature-Controlled Zinc Coordination Polymers: Ionothermal Synthesis, Properties, and Dye Adsorption
Meng-Meng Wang, Zhen Wei, Ling Xu, Bing Liu, Huan Jiao
Index: 10.1002/ejic.201701161
Full Text: HTML
Abstract
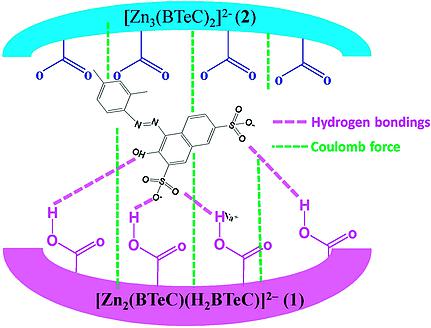
Ionothermal reactions of Zn(NO3)2 with 1,2,4,5-benzenetetracarboxylic acid (H4BTeC) produced two temperature-controlled zinc(II) coordination polymers, [PMI]2[Zn2(BTeC)(H2BTeC)] (1) and [PMI]2[Zn3(BTeC)2]·2H2O (2; PMI = 1-methyl-3-propylimidazolium). The reaction temperature affects the extent of deprotonation of the H4BTeC ligand, leading to different structures and properties. Coordination polymers 1 and 2 show different adsorptions of Ponceau 2R (P2R): 1 strongly adsorbs P2R, whereas 2 exhibits a much weaker adsorption. This behavior can be explained by a synergistic mechanism involving hydrogen-bonding and coulombic interactions, mainly hydrogen bonding at high concentration and coulombic interactions at low concentration of the dye. Ionothermal reactions of Zn(NO3)2 with 1,2,4,5-benzenetetracarboxylic acid (H4BTeC) produced two 3D temperature-controlled coordination polymers, [PMI]2[Zn2(BTeC)(H2BTeC)] (1, PMI = 1-methyl-3-propylimidazolium) and [PMI]2[Zn3(BTeC)2]·2H2O (2). Coordination polymer 1, the main product obtained at 120 °C, strongly adsorbs Ponceau 2R, whereas 2, the controlled product above 170 °C, adsorbs Ponceau 2R much more weakly.
|
Increased Efficiency of Dye‐Sensitized Solar Cells by Incorp...
2018-04-06 [10.1002/ejic.201800123] |
|
Exploring Synthetic Routes to Heteroleptic UIII, UIV, and Th...
2018-04-06 [10.1002/ejic.201800036] |
|
[Co(MeTAA)] Metalloradical Catalytic Route to Ketenes via Ca...
2018-04-06 [10.1002/ejic.201800101] |
|
Copper(I)–Dioxygen Reactivity in the Isolated Cavity of a Na...
2018-04-06 [10.1002/ejic.201800029] |
|
Insight into Solvent Coordination of an Iron Porphyrin Hydro...
2018-03-25 [10.1002/ejic.201800040] |